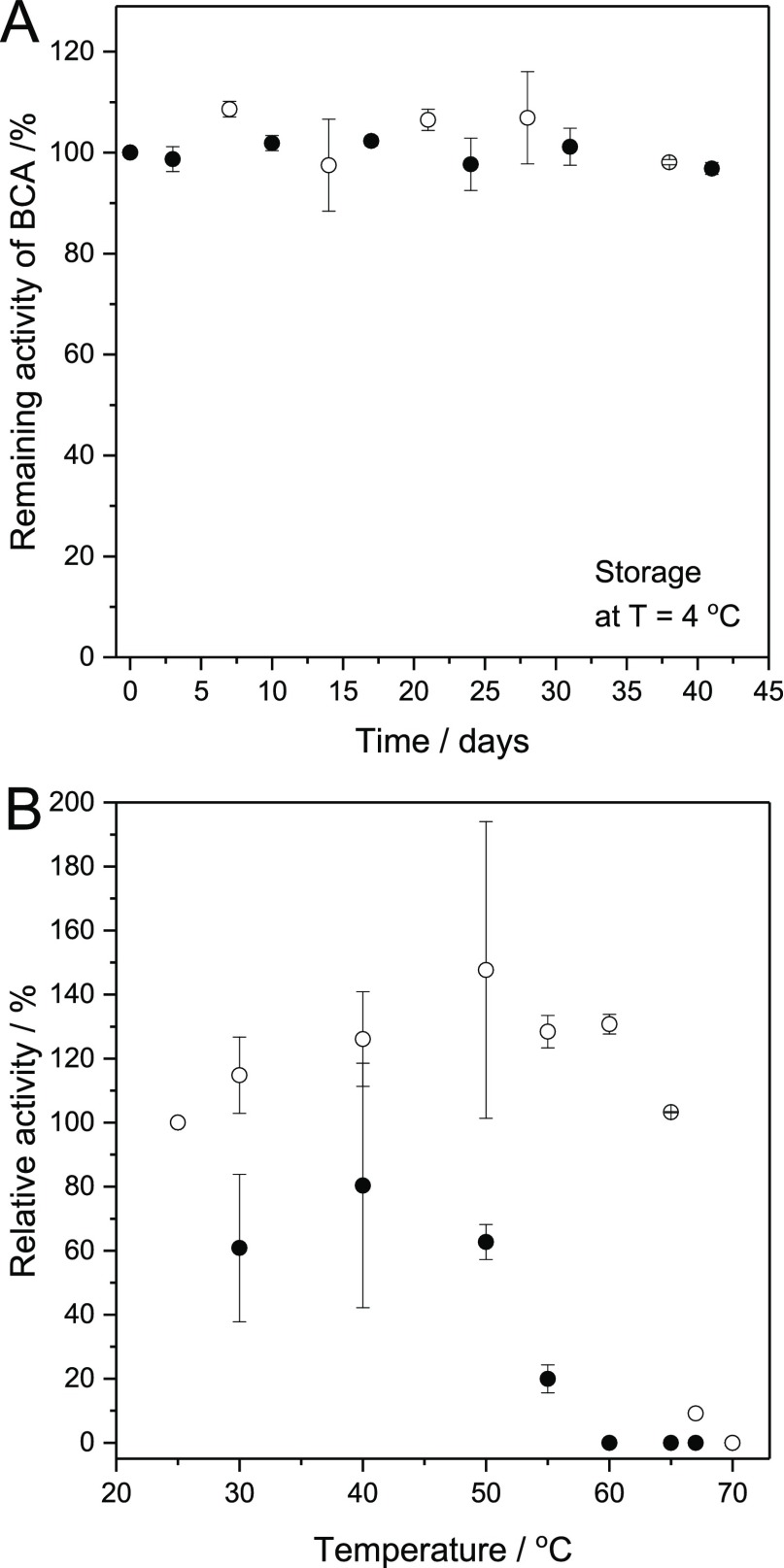
Figure 5.
Storage stability of de-PG2-BAH-BCA (●) and free BCA (○) in aqueous solution at pH = 7.2 for T = 4 °C (A) and 25 °C ≤ T ≤ 70 °C (B). (A) Remaining relative activity of a de-PG21000-BAH175-BCA115 solution (●) and of free BCA (○), which was stored at 4 °C in PB2 (pH = 7.2) in a polypropylene tube at an initial volume of 1.3 mL. The overall concentration of BCA was 29.4 μM. For the activity measurements, aliquots (10 μL) were withdrawn and diluted with 980 μL of PB1 (pH = 7.2), followed by addition of 10 μL of substrate solution (100 mM p-NA in acetonitrile). The total concentration of NaCl during the activity measurement at 25 °C was 0.16 M. The background hydrolysis of 1.0 mM p-NA was also measured in a 0.1 M phosphate buffer solution containing 0.16 M NaCl in the absence of enzyme and then subtracted from the value obtained with the enzyme. The data thus obtained and shown represent mean values ± standard deviations (n = 3). The activity measured before storage was taken as 100%. (B) Effect of temperature on the relative activity of de-PG21000-BAH451-BCA293 (●) and free BCA (○) dissolved in PB3 (pH = 7.2). In both cases, the BCA concentration was about 3.4 μM. The samples were incubated at the indicated temperature for 30 min followed by incubation at 25 °C for 30 min. Then, the BCA activity was measured with 1.0 mM p-NA at 25 °C (see Supporting Information). The measured activity at 25 °C in PB3 without heat treatment was taken as 100%. The rates of hydrolysis in the presence of the conjugate at T ≥ 60 °C and in the presence of free BCA at T = 70 °C were smaller than the rate of the background hydrolysis. In these cases, the relative activity of BCA was taken as zero. The number of measurements n was n = 3 at 25–55 °C for both free BCA and conjugates, and n = 2 at 60–65 °C and n = 1 at 67 °C for free BCA. For the cases of n ≥ 2, the data represent mean values ± standard deviations.