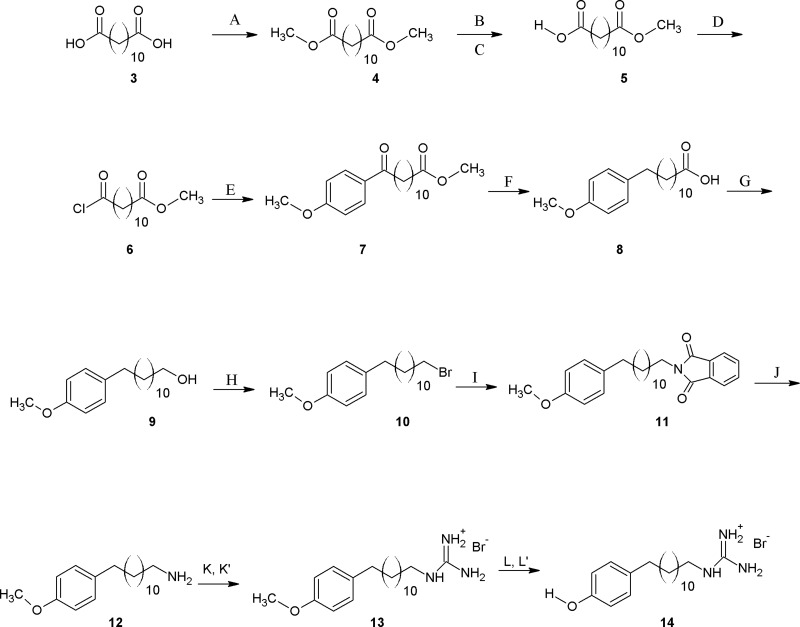
Scheme 3. Overall Synthesis Scheme for (p-Phenolyl) Dodecylguanidinium Bromide (14) and for the Corresponding 15N2-Labeled Compound (14′).
Not shown here is the deprotonation step needed to form the corresponding free-base compounds 15 and 15′. This was carried out as in Scheme 1. Reagents and conditions: (A) reflux, 8 h, H2SO4 (cat), methanol; (B) Ba(OH)2 (1/2 equiv), 24 h, methanol; (C) 2 N HCl, ether; (D) SOCl2, dichloromethane (DCM); (E) AlCl3, anisole, CH2Cl2, DCM; (F) H2NNH2, tBuOK, 48 h, dimethyl sulfoxide (DMSO); (G) LiAlH4, 5 h, THF; (H) PhP3, CBr4, 12 h, DCM; (I) potassium phthalimide, reflux, 2 h, dimethylformamide; (J) H2NNH2, reflux, 12 h, absolute ethanol; (K) S-methyl thiourea hydroiodide, 2 h, absolute ethanol; (K′) S-ethyl thiourea-15N2 hydrobromide, 2 h, absolute ethanol; (L, L′) 48 % HBr, reflux, 6 h.