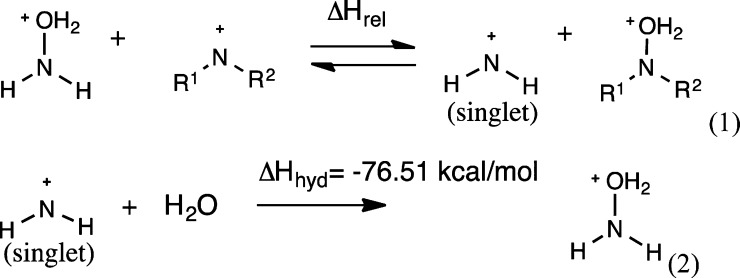
Table 3. Hydration Enthalpies (M06-2x-SMD) for Singlet Nitrenium Ions.
| entry | R1 | R2 | ΔHrel (kcal/mol, 298 K) | ΔHhyd (kcal/mol, 298 K) | |
|---|---|---|---|---|---|
| 1 | H | H | 1 | 0.00 | –76.51a |
| 2 | H–CO | H | 28 | 29.90 | –46.61 |
| 3 | CH3CO | H | 25 | 41.36 | –35.15 |
| 4 | CH3 | H | 2 | 58.60 | –17.91 |
| 5 | CH3 | CH3 | 3 | 64.87 | –11.64 |
| 6 | H–CO | CH=CH2 | 31 | 70.36 | –6.15 |
| 7 | CH3CO | OCH3 | 33 | 74.67 | –1.84 |
| 8 | CH2=CH– | H | 19 | 75.02 | –1.49 |
| 9 | H–CO | OCH3 | 32 | 75.38 | –1.13 |
| 10 | CH3O | H | 25 | 76.64 | 0.13 |
| 11 | CH3O | C(CH3)3 | 27 | 79.53 | 3.02 |
| 12 | CH3O | CH3 | 26 | 80.23 | 3.72 |
| 13 | CH2=CH– | C(CH3)3 | 21 | 82.17 | 5.66 |
| 14 | CH2=CH– | CH3 | 20 | 82.81 | 6.30 |
| 15 | Ph | C(CH3)3 | 24 | 83.79 | 7.28 |
| 16 | Ph | H | 22 | 84.30 | 7.79 |
| 17 | Ph | CH3 | 23 | 88.30 | 11.79 |
| 18 | cyclopropyl | H | 15 | 91.85 | 15.34 |
| 19 | cyclopropyl | CH3 | 16 | 98.41 | 21.90 |
| 20 | cyclobutyl | H | 18 | 99.26 | 22.75 |
| 21 | cyclopropyl | C(CH3)3 | 17 | 101.45 | 24.94 |
A CBS-APNO calculation using an SMD-simulated aqueous solvation.