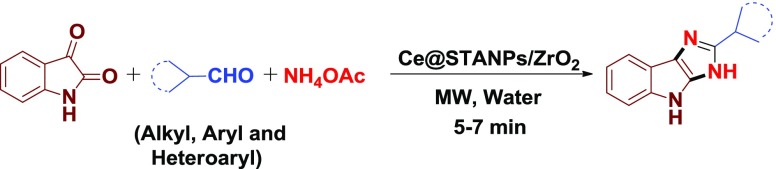
Abstract
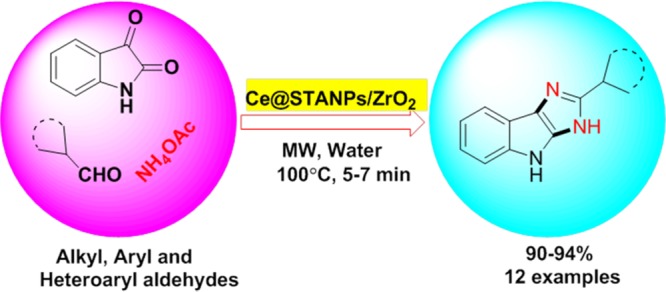
A highly efficient and recyclable catalyst, cerium-immobilized silicotungstic acid nanoparticle-impregnated zirconia (Ce@STANPs/ZrO2), has been synthesized. The catalytic activity of Ce@STANPs/ZrO2 was investigated for the first time in multicomponent synthesis of isatin-based imidazoles under microwave irradiation in water. Ce@STANPs/ZrO2 was used for C=O bond activation in overall reaction to synthesize isatin-based imidazoles. The structure of catalyst was confirmed by characterization techniques, such as Fourier transform infrared (FTIR), scanning electron microscopy (SEM)/energy dispersive X-ray, elemental mapping, transmission electron microscopy, ζ-potential and diffraction light scattering, X-ray diffraction (XRD), thermogravimetric analysis, temperature-programmed desorption-NH3, electron paramagnetic resonance (EPR), and inductively coupled plasma atomic emission spectroscopy (ICP-AES) analyses. The recovered catalyst was found to be efficient up to seventh cycle and was confirmed by FTIR, SEM, XRD, EPR, and ICP-AES analyses. The advantages of the present protocol are recyclability of catalyst, green reaction conditions, excellent yield (94%) of the products, shorter reaction time period (5–7 min), and clean reaction profile.
1. Introduction
In recent years, catalysis has become an attractive methodology to make organic reactions environmentally benign and economically feasible. Heterogeneous catalysts are of great interest because they are easily recoverable from reaction mixture by simple filtration and can be reused.1 Of the known heterogeneous catalysts, heteropoly acids (HPAs) have attracted much attention because of their high acidity, high thermal stability, and favorable redox (acid–base) properties.2 Modification of HPAs by exchanging their protons with metal ions, specially lanthanides, further enhances the catalytic activity in terms of higher acidity, selectivity, and physiochemical properties.3 Among the HPAs, silicotungstic acid (STA) has been widely used as a catalyst in many organic transformations.4 Various metal-doped STA catalysts have also been reported with improved catalytic activity such as stronger acidity and selectivity than that with STA alone.3 Doping of metals on nanoparticles (NPs) further improves the performance of catalyst to a great extent because of their size,5 structure,6 and surface composition.7 These properties enhance the catalytic selectivity,8 activity,9 and lifetime,10 which can be exploited in many industrially important reactions. Further, to achieve high surface area and stability in polar solvents, STA is impregnated on to different supports, such as polymers and silica.11
It is worthy to mention here that among the synthesized organic scaffolds, imidazoles are very important motifs in a number of organic molecules and have gained significant attention due to their varied biological and pharmacological activities, such as antiulcerative agent,12 selective benzodiazepine receptor antagonist,13 proton pump inhibitor,14 platelet coagulation drug in animal and human beings15 (Figure 1), antibacterial agent,16 antitumor agent,17 as pesticides,18 CB1 cannabinoid receptor antagonist,19 modulators of P-glycoprotein-mediated multidrug resistance,20 and as a glucagon receptor.21 Various acidic and basic catalysts have been used in synthesizing substituted imidazoles (Figure 2) such as silica sulfuric acid,22 acetic acid,23 ZrCl4,24 boric acid using ultrasound irradiation,25 Yb(OTf)3,26 potassium dihydrogen phosphate,27 sodium bisulfite,28l-proline,29 and nanozirconia.30 The major drawbacks of most of these protocols are nonrecyclability of catalyst, use of reflux conditions, prolonged time period, use of expensive and toxic catalysts, high temperature, and low product yields.22,23,29 To improve the reaction conditions, keeping in view above-mentioned biological activities of imidazole scaffolds and in continuation of our previous research work,31 we herein, report cerium-immobilized silicotungstic acid nanoparticles-impregnated zirconia (Ce@STANPs/ZrO2) as a catalyst to synthesize a series of isatin-based imidazoles under microwave (MW) irradiation in water.
Figure 1.
Biologically active heterocyclic organic scaffolds bearing imidazole moiety.
Figure 2.
Synthetic methods for imidazoles derived from isatin.
2. Results and Discussion
2.1. Synthesis and Characterization of Catalyst
The catalyst (Ce@STANPs/ZrO2) was synthesized as outlined in Scheme 1. The protons of heteropoly acids are highly acidic and can be exchanged easily by metal ions.3a The driving force for the immobilization of Ce species on STANP therefore is greater stability of Ce@STANPs formed as a result of exchange of protons of STANPs with Ce cations.
Scheme 1. Synthesis of Cerium-Immobilized Silicotungstic Acid Nanoparticle-Impregnated Zirconia (Ce@STANPs/ZrO2).
The synthesized catalyst was characterized by spectral techniques, including Fourier transform infrared (FTIR), scanning electron microscopy (SEM)/energy dispersive X-ray (EDX), transmission electron microscopy (TEM), ζ-potential and diffraction light scattering (DLS), X-ray diffraction (XRD), thermogravimetric analysis, temperature-programmed desorption (TPD)-NH3, electron paramagnetic resonance (EPR), and inductively coupled plasma atomic emission spectroscopy (ICP-AES) analyses.
2.1.1. FTIR Analysis
FTIR spectrum of ZrO2 (Figure S1A) showed a broad band at 3412 cm–1 due to asymmetric stretching vibration of −OH group. Two bands at 1633 and 1446 cm–1 correspond to −(H–O–H) bending and −(O–H–O) bending vibrations. A weak band in the fingerprint region at 583 cm–1 was assigned for Zr–O–H bond.32 In IR spectrum of STANPs (Figure S1B), a broad band at 3424 cm–1 had appeared, which was attributed to −OH group, whereas Si–O stretching band was present at 1080 cm–1. The stretching mode of W=O bond was present at 981 cm–1, whereas stretching modes of edge sharing (W–Oe–W) and corner sharing (W–Oc–W) units appeared between 800 and 900 cm–1.33 When cerium was doped in STANPs, a characteristic band of Ce–O appeared at 621 cm–1 34 along with other bands due to STANPs and ZrO2 (Figure S1C). When Ce@STANPs was supported on ZrO2, a broad peak at 1054 cm–1 had appeared due to merging of stretching and bending modes of vibrations of STANPs and ZrO2 at 1080, 981, 895, and 875, as these values lie closely in the same range with respect to each other (Figure S1D).
2.1.2. SEM/EDX Analysis
The surface morphology of the zirconia, fresh catalyst, and recycled catalyst was determined by scanning electron microscope (SEM) analysis and is shown in Figure 3. The SEM image of the catalyst showed an even distribution of Ce@STANPs on the surface of zirconia (Figure 3a). The presence of Ce, Zr, W, Si, and O elements in the catalyst was confirmed by energy dispersive X-ray (EDX) analysis (Figure 4a). Elemental mapping of the catalyst (Figure 4b) confirmed the uniform distribution of all elements (i.e., Ce, W, Si, and O) on the surface of zirconia. Further, mapping of individual elements, viz., O (Figure 4c), Zr (Figure 4d), Si (Figure 4e), W (Figure 4f), and Ce (Figure 4g) was also shown. These images confirmed formation of the expected catalytic system, Ce@STANPs/ZrO2.
Figure 3.
SEM image of (a) fresh catalyst (Ce@STANPs/ZrO2), (b) recycled catalyst after the seventh run, and (c) zirconia.
Figure 4.
(a) EDX analysis of catalyst (Ce@STANPs/ZrO2) (b) and elemental mapping of catalyst showing all elements, (c) mapping of oxygen, (d) mapping of zirconium, (e) mapping of silicon, (f) mapping of tungsten, and (g) mapping of cerium.
2.1.3. TEM Analysis
TEM images of ZrO2, STANPs, and Ce@STANPs/ZrO2 catalyst are shown in Figure S2. The particles of ZrO2 were obtained free from aggregation, and most of the particles were well separated and have defined grain boundaries, as shown in Figure S2a. The TEM image of STANPs clearly indicated the well-separated particles (Figure S2b) with particle size of 4–10 nm, as shown in the particle distribution curve (Figure S2d). The average particle size was found as ∼6–7 nm. When STANPs were supported on ZrO2, particles of STANPs were attached on to the surface of ZrO2 (Figure S2c). The TEM image of the catalyst (Ce@STANPs/ZrO2) also confirmed that the particles were free from agglomeration.
The diffraction light scattering (DLS) analysis of the catalyst showed two peaks at different positions, confirming particle sizes as ∼4–10 and ∼30–100 nm for STANPs and ZrO2, respectively (Figure 5).
Figure 5.

DLS analysis of the catalyst (Ce@STANPs/ZrO2).
2.1.4. ζ-Potential
To check the stability of catalyst, ζ-potential of the catalyst was performed and found to be −43.08 mV, which showed that the catalyst was fairly stable because the minimum value for the nanoparticle samples to be stable is reported as ±30 mV.35
2.1.5. TG Analysis
Thermal stability of the catalyst was observed by thermogravimetric (TG) analysis. The TG curve (Figure S3) showed two-stage decomposition due to changes occurring in the catalyst at different temperatures. The curve showed a weight loss of 5.82% at 52.40 °C due to surface-physisorbed water. Another weight loss of about 2.04% at 385 °C can be attributed to the decomposition of the Keggin structure of STA accompanied by removal of water.36
2.1.6. XRD Analysis
The XRD analysis of STANPs, Ce@STANPs, ZrO2, fresh catalyst Ce@STANPs/ZrO2, and recycled catalyst was shown in Figure S4. A broad peak in the range of 20–30° was attributed to Si–O–Si bond, which was the main component of STANPs (Figure S4a).37 When cerium was doped in STANPs, some other diffraction peaks in the range of 50–60° appeared, which may be due to Ce (Figure S4b). In XRD pattern of ZrO2, the diffraction peaks that appeared at around 2θ = 30, 35, 40, 50, and 60° belonged to (111), (200), (220), and (311) planes (Figure S4c).38 When Ce@STANPs was supported on ZrO2, the same diffraction pattern was obtained as that for ZrO2, with some additional peaks in the range of 50–60° corresponding to Ce.
2.1.7. TPD-NH3 Analysis
The total acidity and presence of acidic sites on the surface of the catalyst was measured by TPD-NH3 analysis. The TPD pattern of STANPs/ZrO2 (Figure S5a) and Ce@STANPs/ZrO2 (Figure S5b) showed two peaks at 240 and 570 °C, which revealed the desorption of basic molecules from acidic sites present on the surface of the catalyst. The acidic property of the catalyst enhanced when cerium was doped to STANPs/ZrO2 (Figure S5).
2.1.8. EPR Analysis
To get insight of the presence of paramagnetic cerium ion in the catalyst, X-band EPR spectrum of the powdered sample was recorded. The observed pattern of the spectrum was anisotropic in nature, confirming the presence of Ce3+ (4f1 system) (Figure S6). The calculated g value (2.028) indicated the presence of one unpaired electron in the metal ion of the catalyst.39
2.1.9. ICP-AES Analysis
ICP-AES analysis was performed to confirm the actual concentration of cerium incorporated in the catalyst. The weight % of cerium was found to be 8.42%, which corresponded to the loading amount of 0.6 mmol/g of catalyst.
2.2. Evaluation of Catalytic Activity of Ce@STANPs/ZrO2 for Synthesizing Isatin-Based Imidazoles
The optimum reaction conditions were developed using several parameters, such as screening of different catalysts and solvents, catalyst loading on different supports, and effect of catalyst amount and temperature on the model reaction. The reaction of isatin 1, benzaldehyde 2d, and ammonium acetate 3, affording 2-phenyl-3,4-dihydroimidazo[4,5-b]indole 4d, was chosen as the model reaction (Scheme 2).
Scheme 2. Synthesis of 4d.
Efficiency of the catalyst on the model reaction was investigated by a set of experiments using different catalysts. First of all, the model reaction was carried out without any catalyst and trace amount of the product was obtained after a long period of time (Table 1, entry 1). Then, lanthanide salts were used as catalyst, which afforded moderate yield of the product (Table 1, entries 2–4). Among lanthanide salts, cerium chloride showed better catalytic activity (Table 1, entry 4). However, catalytic activity was enhanced and yield was improved with heteropoly acids, phosphotungstic acid (PTA) and STA (Table 1, entries 5 and 6). Use of NPs (STANPs) further accelerated the rate of reaction with improved yield (Table 1, entry 7). Doping of lanthanide-metal salts on STANPs, i.e., use of Nd@STANPs, La@STANPs, and Ce@STANPs, reduced the time period and afforded good yields of the product (Table 1, entries 8–10). However, Ce@STANPs showed good result among the three (Table 1, entry 10). Concerning the surface area of the catalyst, support materials were also used to optimize the reaction conditions. Use of ZrO2 as support on STANPs enhanced the yield in comparison with STANPs alone (Table 1, entry 11). On the basis of these observations, the model reaction was carried out using Ce@STANPs with different support materials such as SiO2, Al2O3, and ZrO2 (Table 1, entries 12–14). It was found that the best result (5 min, 94% yield) was obtained when cerium was used as a doping metal and ZrO2 was used as a support material on STANPs, i.e., using Ce@STANPs/ZrO2 as catalyst on the model reaction (Table 1, entry 12).
Table 1. Effect of Different Catalysts on Model Reactiona.
| entry | catalyst | time (min)b | yieldc (%) |
|---|---|---|---|
| 1 | no catalyst | 30 | trace |
| 2 | NdCl3 | 21 | 46 |
| 3 | LaCl3 | 18 | 53 |
| 4 | CeCl3 | 12 | 62 |
| 5 | PTA | 12 | 72 |
| 6 | STA | 10 | 75 |
| 7 | STANPs | 9 | 78 |
| 8 | Nd@STANPs | 12 | 81 |
| 9 | La@STANPs | 13 | 80 |
| 10 | Ce@STANPs | 9 | 88 |
| 11 | STANPs/ZrO2 | 15 | 81 |
| 12 | Ce@STANPs/ZrO2 | 5 | 94 |
| 13 | Ce@STANPs/SiO2 | 12 | 91 |
| 14 | Ce@STANPs/Al2O3 | 11 | 90 |
Reaction conditions: isatin 1 (2 mmol), benzaldehyde 2d (2 mmol), ammonium acetate 3 (5 mmol), catalysts (80 mg), water, T = 100 °C, MW.
Reaction progress monitored by thin-layer chromatography (TLC).
Isolated yield.
To investigate the effect of solvents on the model reaction, different green organic solvents were used in various conditions. Using ethanol and isopropanol, good results were obtained (Table 2, entries 1 and 2). However, yield was slightly decreased in the presence of poly(ethylene glycol) (PEG)-200, PEG-400, and PEG-600 (Table 2, entries 3–5). When water was mixed with isopropanol and ethanol, i.e., using isopropanol/water and ethanol/water, comparatively good results with improved yield were obtained (Table 2, entries 6 and 7). Use of water as solvent showed better results among all green solvents used to optimize the reaction conditions in terms of excellent yield (94%) and a short reaction time period (5 min) (Table 2, entry 8).
Table 2. Effect of Solvents on Model Reactiona.
| entry | solvent | time (min)b | yieldc (%) |
|---|---|---|---|
| 1 | ethanol | 21 | 74 |
| 2 | isopropanol | 28 | 69 |
| 3 | PEG-200 | 22 | 56 |
| 4 | PEG-400 | 25 | 54 |
| 5 | PEG-600 | 23 | 59 |
| 6 | isopropanol/water | 16 | 75 |
| 7 | ethanol/water | 14 | 81 |
| 8 | water | 5 | 94 |
Reaction conditions: isatin 1 (2 mmol), benzaldehyde 2d (2 mmol), ammonium acetate 3 (5 mmol), (Ce@STANPs/ZrO2) (80 mg), solvents, T = 100 °C, MW.
Reaction progress monitored by TLC.
Isolated yield.
To see the efficiency of catalyst loading on support, variable amounts of Ce@STANPs were supported on ZrO2. It was observed that yield was improved with increasing amount of Ce@STANPs on support material (Table S1). However, excellent result was obtained when 20% (w/w) of Ce@STANPs was supported on ZrO2 (Table S1, entry 4). Further increasing the amount of Ce@STANPs on support, slightly reduced the yield of the product (Table S1, entry 5). From this observation, 20% (w/w) Ce@STANPs was supported on ZrO2 for better catalytic activity in multicomponent synthesis of isatin-based imidazoles.
The amount of catalyst suitable to catalyze the model reaction had been investigated by varying the amount of Ce@SNPs/ZrO2 to catalyze the model reaction. The result showed that yield was improving sequentially by increasing the amount of catalyst from 20 to 80 mg (Table S2). Further increasing the loading amount of catalyst from 80 to 100 mg did not show any remarkable change; yield was the same (94%), and time was increased from 5 to 6 min (Table S2, entries 5 and 6). Considering this observation, 80 mg of catalyst was chosen for the catalytic activity.
The model reaction was also examined with different temperatures to know the effect of temperature on multicomponent synthesis of isatin-based imidazoles. Considering general kinetics, both rate of reaction and product yield were improved by increasing the temperature from 70 to 100 °C (Table S3). Encouraged by this result, the multicomponent reaction was performed at 100 °C to get the optimal result (Table S3, entry 5).
2.3. Comparison of Efficiency of Catalyst with Previously Reported Procedures
The efficiency of Ce@STANPs/ZrO2 in multicomponent synthesis of isatin-based imidazoles from isatin, aldehydes, and ammonium acetate was seen by comparing the previously reported results (Table 3). Although product yield was reported as 74–96% by ref (29), the drawback of this protocol was limited use of l-proline (Table 3, entry 2) because l-proline cannot be recycled. The superiority of Ce@STANPs/ZrO2 over nanozirconia was confirmed by decreasing the time from 30 to 5–7 min, temperature from 110 to 100 °C and improving yield from 78–93 to 90–94% (Table 3, entries 3 and 5).
Table 3. Comparison of Results Obtained in Various Conditions for Multicomponent Synthesis of Isatin-Based Imidazoles.
| entry | catalyst | condition | time | yield (%) | refs |
|---|---|---|---|---|---|
| 1 | no catalyst | acetic acid/MW | 12–22 min | 67–93 | (23) |
| 2 | l-proline | ethanol/reflux | 6–10 h | 85–90 | (29) |
| 3 | l-proline | ethanol/RT, ultrasound irradiation | 10–17.5 min | 74–96 | (29) |
| 4 | nanozirconia | solvent free/110 °C | 30 min | 78–93 | (30) |
| 5 | Ce@STANPs/ZrO2 | water/100 °C, MW | 5–7 | 90–94 | this work |
2.4. Catalytic Reaction
The isatin-based imidazoles were synthesized in the presence of Ce@STANPs/ZrO2 under microwave irradiation in water at 100 °C (Scheme 3; Table 4).
Scheme 3. General Procedure for the Synthesis of Isatin-Based Imidazoles.
Table 4. Synthesis of Isatin-Based Imidazoles (4a–l) Using Ce@STANPs/ZrO2 under Conventional Method and Microwave Irradiation.
Newly synthesized compound.
Reported compound.30
Reaction progress monitored by TLC.
Isolated yield.
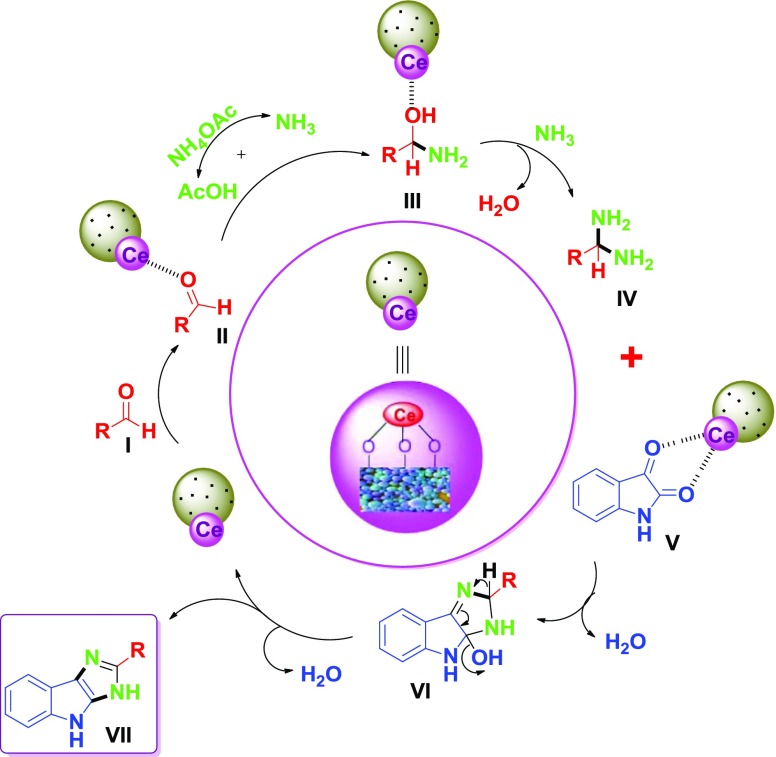
2.5. Reaction Mechanism
The plausible mechanism for the synthesis of isatin-based imidazoles is shown in Scheme 4. The carbonyl group of substituted aldehydes I was activated with cerium-doped catalyst (Ce@STANPs/ZrO2) to form activated aldehydes II. This accelerated the nucleophilic attack of ammonia (from dissociation of ammonium acetate) on II to form intermediate III. Another molecule of ammonia reacted with III and liberated a water molecule to form intermediate IV that reacted with activated isatin molecule V to afford intermediate VI. Finally, liberation of a water molecule form VI afforded product VII, separating the catalyst.
Scheme 4. Plausible Mechanism for the Synthesis of 4a–l.
2.6. Recycling Study of Catalyst
The recycling study of the catalyst was done using the model reaction of isatin 1, benzaldehyde 2d, ammonium acetate 3, and 80 mg of Ce@STANPs/ZrO2 in water to afford 4d. The reaction mixture was heated at 100 °C, and after the completion of reaction, the catalyst was recovered by extracting the mixture with ethyl acetate, followed by filtration. The catalyst was reused for subsequent cycles and was found to retain the catalytic activity up to seventh cycle with over 88% conversion of the substrate to the product (Table S4, Figure S7).
The recycled catalyst was characterized by FTIR, SEM, EPR, XRD, and ICP-AES analyses. There was no appreciable change in the FTIR spectrum of the recovered catalyst. All characteristic bands (1621, 620, and 547 cm–1) were retained in the spectrum, confirming that there was no loss in the structure of the recycled catalyst (Figure S1E). The SEM image (Figure 3b) showed that surface morphology of the recovered catalyst did not change after the seventh cycle. The XRD pattern of the recycled catalyst (Figure S4e) remained the same, showing no structural change in the catalyst after the seventh cycle. EPR study of the recycled catalyst confirmed that oxidation state of cerium (Ce3+) did not change during the course of reaction (all stages) because the value of g = 2.028 (Figure S6a) was approximately the same (g = 2.046) (Figure S6b) in the recovered catalyst. The ICP-AES analysis showed almost the same percentage of Ce after the seventh cycle, indicating that no leaching of Ce occurred during the reaction course.
3. Conclusions
In conclusion, we have developed a simple, efficient, and environmentally benign method for the synthesis of isatin-based imidazoles, employing Ce@STANPs/ZrO2 as a recyclable heterogeneous catalyst for the first time. The advantageous features of the present protocol include reusability of the catalyst up to the seventh cycle, excellent product yield, shorter reaction time period, and green reaction condition.
4. Experimental Section
4.1. Preparation of Catalyst
The catalyst was prepared in three steps according to Scheme 1. In the first step, STANPs were prepared by a slightly modified procedure reported by Izumi.40 A mixture of STA (1.439 g), water (36 mL), 1-butanol (15 mL), and tetraethyl orthosilicate (0.2 mol) was stirred for 3 h at 80 °C. This mixture was dehydrated at the same temperature to obtain dried hydrogel. The hydrogel was then extracted with methanol in Sohxlet apparatus for 2 days. STANPs obtained were dried at 100 °C for 3 h. In the second step, 20% aqueous solution of CeCl3·7H2O (5 mL) was added with STANPs in water (20 mL) and stirred for 2 h at room temperature to obtain cerium-immobilized silicotungstic acid nanoparticles (Ce@STANPs). Zirconia-supported Ce@STANPs were prepared by employing impregnation method. As confirmed from previous studies that 15% (w/w) loading of HPA on zirconia showed better catalytic activity,41 dried zirconia powder was suspended in 15% (w/w) methanolic solution of Ce@STANPs and stirred for 2 h and excess methanol was evaporated to dryness. The resulting sample was dried at 120 °C for 3 h to obtain the final catalyst Ce@STANPs/ZrO2.
4.2. General Procedure for the Synthesis of Isatin-Based Imidazoles (4a–l) under Conventional Heating
A mixture of isatin 1 (3 mmol), aliphatic/aromatic/heteroaromatic aldehydes 2a–l (3 mmol), ammonium acetate 3 (10 mmol), and Ce@STANPs/ZrO2 (80 mg) in water was heated at 100 °C for an appropriate time period. Reaction progress was monitored through TLC. After completion of reaction (monitored by TLC), reaction mixture was kept at room temperature and after reaching 25 °C, acidified with 1 N HCl to get precipitate of a different color. The precipitate was then extracted with ethyl acetate, washed with double distilled water, dried over anhydrous sodium sulfate, and evaporated under reduced pressure to get crude product, which was then recrystallized with ethanol to get pure product 4a–l. The catalyst in aqueous phase was filtered and washed with ethanol for further catalytic use.
4.3. General Procedure for the Synthesis of Isatin-Based Imidazoles (4a–l) under Microwave Irradiation
A mixture of isatin 1 (3 mmol), aromatic/heteroaromatic/aliphatic aldehydes 2a–l (3 mmol), ammonium acetate 3 (10 mmol), and Ce@STANPs/ZrO2 (80 mg) in water was taken in G30 vial and irradiated with microwaves at 100 °C for 5–7 min. After each 60 s, intermittent cooling was done and meanwhile reaction mixture was thoroughly mixed. Reaction progress was monitored by TLC. After completion of reaction, reaction mixture was withdrawn from the microwave oven, allowed to cool, and acidified with 1 N HCl to get precipitate. The precipitate obtained was then extracted with ethyl acetate, washed with double distilled water, dried over anhydrous sodium sulfate, and evaporated under reduced pressure to obtain crude product, which was then recrystallized with ethanol to get pure product 4a–l. The catalyst in aqueous phase was filtered and washed with ethanol for further catalytic use.
Acknowledgments
Financial assistance in the form of Major Research Project (No. CST/SERPD/D-283) from CST-UP is gratefully acknowledged. The authors would also like to acknowledge DRS II (New Delhi), USIF, A.M.U. for SEM/EDX and TEM analyses and Department of Physics, A.M.U., Aligarh for XRD analysis.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01043.
FTIR, EPR, and TPD-NH3 spectra; XRD analysis; TEM images; spectral data of newly synthesized compounds; effect of Ce@STANPs; recycling study of catalyst (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Van Santen R. A. Complementary structure sensitive and insensitive catalytic relationships. Acc. Chem. Res. 2009, 42, 57–66. 10.1021/ar800022m. [DOI] [PubMed] [Google Scholar]
- Zhang D. Y.; Duan M. H.; Yao X. H.; Fu Y. J.; Zu Y. G. Preparation of a novel cellulose-based immobilized heteropoly acid system and its application on the biodiesel production. Fuel 2016, 172, 293–300. 10.1016/j.fuel.2015.12.020. [DOI] [Google Scholar]
- a Corma A.; Martınez A.; Martınez C. Acidic Cs+, NH4+, and K+ salts of 12-tungstophosphoric acid as solid catalysts for isobutane/2-butene alkylation. J. Catal. 1996, 164, 422–432. 10.1006/jcat.1996.0398. [DOI] [Google Scholar]; b Haider M. H.; Dummer N. F.; Zhang D.; Miedziak P.; Davies T. E.; Taylor S. H.; Willock D. J.; Knight D. W.; Chadwick D.; Hutchings G. J. Rubidium-and caesium-doped silicotungstic acid catalysts supported on alumina for the catalytic dehydration of glycerol to acrolein. J. Catal. 2012, 286, 206–213. 10.1016/j.jcat.2011.11.004. [DOI] [Google Scholar]
- a Kim Y. T.; Jung K. D.; Park E. D. Gas-phase dehydration of glycerol over supported silicotungstic acids catalysts. Bull. Korean Chem. Soc. 2010, 31, 3283–3290. 10.5012/bkcs.2010.31.11.3283. [DOI] [Google Scholar]; b Li H.; Yin H.; Jiang T.; Hu T.; Wu J.; Wada Y. Cyclodehydration of 1, 4-butanediol to tetrahydrofuran catalyzed by supported silicotungstic acid. Catal. Commun. 2006, 7, 778–782. 10.1016/j.catcom.2006.02.028. [DOI] [Google Scholar]
- Che M.; Bennett C. O. The influence of particle size on the catalytic properties of supported metals. Adv. Catal. 1989, 36, 55–172. 10.1016/S0360-0564(08)60017-6. [DOI] [Google Scholar]
- Somorjai G. A. The structure sensitivity and insensitivity of catalytic reactions in light of the adsorbate induced dynamic restructuring of surfaces. Catal. Lett. 1991, 7, 169–182. 10.1007/BF00764500. [DOI] [Google Scholar]
- Sajkowski D. J.; Boudart M. Structure sensitivity of the catalytic oxidation of ethene by silver. Catal. Rev.: Sci. Eng. 1987, 29, 325–360. 10.1080/01614948708078611. [DOI] [Google Scholar]
- Somorjai G. A.; McCrea K. Roadmap for catalysis science in the 21st century: a personal view of building the future on past and present accomplishments. Appl. Catal., A 2001, 222, 3–18. 10.1016/S0926-860X(01)00825-0. [DOI] [Google Scholar]
- Ma Z.; Zaera F.. Heterogeneous Catalysis by Metals. In Encyclopedia of Inorganic Chemistry, 2nd ed.; King R. B., Ed.; John Wiley & Sons: New York, 2005; pp 1768–1784. [Google Scholar]
- Joo S. H.; Park J. Y.; Tsung C. K.; Yamada Y.; Yang P.; Somorjai G. A. Thermally stable Pt/mesoporous silica core–shell nanocatalysts for high-temperature reactions. Nat. Mater. 2009, 8, 126–131. 10.1038/nmat2329. [DOI] [PubMed] [Google Scholar]
- Varisli D.; Dogu T.; Dogu G. Novel mesoporous nanocomposite WO x-silicate acidic catalysts: ethylene and diethylether from ethanol. Ind. Eng. Chem. Res. 2009, 48, 9394–9401. 10.1021/ie8008154. [DOI] [Google Scholar]
- Brimblecombe R. W.; Duncan W. A. M.; Durant G. J.; Emmett J. C.; Ganellin C. R.; Parsons M. E. Cimetidine—a non-thiourea H2-receptor antagonist. J. Int. Med. Res. 1975, 3, 86–92. 10.1177/030006057500300205. [DOI] [Google Scholar]
- Hunkeler W.; Möhler H.; Pieri L.; Polc P.; Bonetti E. P.; Cumin R.; Schaffner R.; Haefely W. Selective antagonists of benzodiazepines. Nature 1981, 290, 514–516. 10.1038/290514a0. [DOI] [PubMed] [Google Scholar]
- Tanigawara Y.; Aoyama N.; Kita T.; Shirakawa K.; Komada F.; Kasuga M.; Okumura K. CYP2C19 genotype–related efficacy of omeprazole for the treatment of infection caused by Helicobacter pylori. Clin. Pharmacol. Ther. 1999, 66, 528–534. 10.1016/S0009-9236(99)70017-2. [DOI] [PubMed] [Google Scholar]
- Abrahams S. L.; Hazen R. J.; Batson A. G.; Phillips A. P. Trifenagrel: a chemically novel platelet aggregation inhibitor. J. Pharmacol. Exp. Ther. 1989, 249, 359–365. [PubMed] [Google Scholar]
- Antolini M.; Bozzoli A.; Ghiron C.; Kennedy G.; Rossi T.; Ursini A. Analogues of 4, 5-bis (3, 5-dichlorophenyl)-2-trifluoromethyl-1H-imidazole as potential antibacterial agents. Bioorg. Med. Chem. Lett. 1999, 9, 1023–1028. 10.1016/S0960-894X(99)00112-2. [DOI] [PubMed] [Google Scholar]
- Wang L.; Woods K. W.; Li Q.; Barr K. J.; McCroskey R. W.; Hannick S. M.; Gherke L.; Credo R. B.; Hui Y. H.; Marsh K.; Warner R.; et al. Potent, orally active heterocycle-based combretastatin A-4 analogues: synthesis, structure– activity relationship, pharmacokinetics, and in vivo antitumor activity evaluation. J. Med. Chem. 2002, 45, 1697–1711. 10.1021/jm010523x. [DOI] [PubMed] [Google Scholar]
- Maier T.; Schmierer R.; Bauer K.; Bieringer H.; Buerstell H.; Sachse B.. 1-Substituted Imidazole-5-carboxylic Acid Derivatives, their Preparation and their Use as Biocides. U.S. Patent US4820335A, 1989.
- Eyers P. A.; Craxton M.; Morricel N.; Cohen P.; Goedert M. Conversion of SB 203580-insensitive MAP kinase family members to drug-sensitive forms by a single amino-acid substitution. Chem. Biol. 1998, 5, 321–328. 10.1016/S1074-5521(98)90170-3. [DOI] [PubMed] [Google Scholar]
- Newman M. J.; Rodarte J. C.; Benbatoul K. D.; Romano S. J.; Zhang C.; Krane S.; Moran E. J.; Uyeda R. T.; Dixon R.; Guns E. S.; Mayer L. D. Discovery and characterization of OC144-093, a novel inhibitor of P-glycoprotein-mediated multidrug resistance. Cancer Res. 2000, 60, 2964–2972. [PubMed] [Google Scholar]
- de Laszlo S. E.; Hacker C.; Li B.; Kim D.; MacCoss M.; Mantlo N.; Pivnichny J. V.; Colwell L.; Koch G. E.; Cascieri M. A.; Hagmann W. K. Potent, orally absorbed glucagon receptor antagonists. Bioorg. Med. Chem. Lett. 1999, 9, 641–646. 10.1016/S0960-894X(99)00081-5. [DOI] [PubMed] [Google Scholar]
- Shaabani A.; Rahmati A. Silica sulfuric acid as an efficient and recoverable catalyst for the synthesis of trisubstitutedimidazoles. J. Mol. Catal. A: Chem. 2006, 249, 246–248. 10.1016/j.molcata.2006.01.006. [DOI] [Google Scholar]
- Kumar N.; Sharma P. K.; Garg V. K.; Singh P. Synthesis and anticonvulsant activity of novel substituted phenyl indoloimidazole derivatives. Curr. Res. Chem. 2011, 3, 114–120. 10.3923/crc.2011.114.120. [DOI] [Google Scholar]
- Sharma G. V. M.; Jyothi Y.; Lakshmi P. S. Efficient Room-Temperature Synthesis of Tri- and Tetrasubstituted Imidazoles Catalyzed by ZrCl4. Synth. Commun. 2006, 36, 2991–3000. 10.1080/00397910600773825. [DOI] [Google Scholar]
- Shelke S. N.; Mhaske G. R.; Bonifácio V. D.; Gawande M. B. Green synthesis and anti-infective activities of fluorinated pyrazoline derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 5727–5730. 10.1016/j.bmcl.2012.06.072. [DOI] [PubMed] [Google Scholar]
- Wang L. M.; Wang Y. H.; Tian H.; Yao Y. F.; Shao J. H.; Liu B. Ytterbium triflate as an efficient catalyst for one-pot synthesis of substituted imidazoles through three-component condensation of benzil, aldehydes and ammonium acetate. J. Fluorine Chem. 2006, 127, 1570–1573. 10.1016/j.jfluchem.2006.08.005. [DOI] [Google Scholar]
- Joshi R. S.; Mandhane P. G.; Shaikh M. U.; Kale R. P.; Gill C. H. Potassium dihydrogen phosphate catalyzed one-pot synthesis of 2, 4, 5-triaryl-1H-imidazoles. Chin. Chem. Lett. 2010, 21, 429–432. 10.1016/j.cclet.2009.11.012. [DOI] [Google Scholar]
- Sangshetti J. N.; Kokare N. D.; Kotharkar S. A.; Shinde D. B. Sodium bisulfite as an efficient and inexpensive catalyst for the one-pot synthesis of 2, 4, 5-triaryl-1H-imidazoles from benzil or benzoin and aromatic aldehydes. Monatsh. Chem. 2008, 139, 125–127. 10.1007/s00706-007-0766-3. [DOI] [Google Scholar]
- a Dianati H. R.; Nazif A. R.; Salimi S.; Teymori V. New three-Component Protocol for the Synthesis of imidazo[4,5- b] indoles. Res. J. Chem. Environ. Sci. 2014, 2, 41–44. [Google Scholar]; b Damavandi S.; Sandaroos R. L-Proline-catalyzed three-component synthesis of condensed imidazoles. Arabian J. Chem. 2016, 9, S1138–S1143. 10.1016/j.arabjc.2011.12.004. [DOI] [Google Scholar]
- Bajpai S.; Singh S.; Srivastava V. Nano zirconia catalysed one-pot synthesis of some novel substituted imidazoles under solvent-free conditions. RSC Adv. 2015, 5, 28163–28170. 10.1039/C4RA16211K. [DOI] [Google Scholar]
- a Siddiqui S.; Khan M. U.; Siddiqui Z. N. Synthesis, Characterization, and Application of Silica-Supported Copper-Doped Phosphotungstic Acid in Claisen–Schmidt Condensation. ACS Sustainable Chem. Eng. 2017, 5, 7932–7941. 10.1021/acssuschemeng.7b01467. [DOI] [Google Scholar]; b Ahmed N.; Siddiqui Z. N. Cerium Supported Chitosan as an Efficient and Recyclable Heterogeneous Catalyst for Sustainable Synthesis of Spiropiperidine Derivatives. ACS Sustainable Chem. Eng. 2015, 3, 1701–1707. 10.1021/acssuschemeng.5b00223. [DOI] [Google Scholar]; c Ahmed N.; Tarannum S.; Siddiqui Z. N. Dy/chitosan: a highly efficient and recyclable heterogeneous nano catalyst for the synthesis of hexahydropyrimidines in aqueous media. RSC Adv. 2015, 5, 50691–50700. 10.1039/C5RA08160B. [DOI] [Google Scholar]
- Tarannum S.; Siddiqui Z. N. Synthesis, characterization and catalytic evaluation of BiCl3-ZrO2 for the synthesis of novel pyrazolyl chalcones. J. Mol. Catal. A: Chem. 2014, 394, 262–273. 10.1016/j.molcata.2014.07.016. [DOI] [Google Scholar]
- Masteri-Farahani M.; Modarres M. Wells-Dawson heteropoly acid immobilized inside the nanocages of SBA-16 with ship-in-a-bottle method: A new recoverable catalyst for the epoxidation of olefins. J. Mol. Catal. A: Chem. 2016, 417, 81–88. 10.1016/j.molcata.2016.03.016. [DOI] [Google Scholar]
- Lai Y. M.; Liang X. F.; Yang S. Y.; Wang J. X.; Cao L. H.; Dai B. Raman and FTIR spectra of iron phosphate glasses containing cerium. J. Mol. Struct. 2011, 992, 84–88. 10.1016/j.molstruc.2011.02.049. [DOI] [Google Scholar]
- Komalam A.; Muraleegharan L. G.; Subburaj S.; Suseela S.; Babu A.; George S. Designed plasmonic nanocatalysts for the reduction of eosin Y: absorption and fluorescence study. Int. Nano Lett. 2012, 2, 26 10.1186/2228-5326-2-26. [DOI] [Google Scholar]
- Villabrille P.; Romanelli G.; Vázquez P.; Cáceres C. Vanadium-substituted Keggin heteropolycompounds as catalysts for ecofriendly liquid phase oxidation of 2, 6-dimethylphenol to 2, 6-dimethyl-1, 4-benzoquinone. Appl. Catal., A 2004, 270, 101–111. 10.1016/j.apcata.2004.04.028. [DOI] [Google Scholar]
- Isahak W. N. R. W.; Roslam W. N.; Manal I.; Nordin N. M.; Hamzah N.; Ghoreishi K. B.; Mohd Jahim J.; Yarmo M. A. Synthesis and characterization of silicotungstic acid nanoparticles via sol gel technique as a catalyst in esterification reaction. Adv. Mater. Res. 2012, 364, 266–271. 10.4028/www.scientific.net/AMR.364.266. [DOI] [Google Scholar]
- a Elshazly E. S.; Abdelal O. A. Nickel stabilized zirconia for SOFCs: synthesis and characterization. Int. J. Metall. Eng. 2012, 1, 130–134. 10.5923/j.ijmee.20120106.07. [DOI] [Google Scholar]; b Maridurai T.; Balaji D.; Sagadevan S. Synthesis and Characterization of Yttrium Stabilized Zirconia Nanoparticles. Mater. Res. 2016, 19, 812–816. 10.1590/1980-5373-MR-2016-0196. [DOI] [Google Scholar]
- Dutta P.; Pal S.; Seehra M. S.; Shi Y.; Eyring E. M.; Ernst R. D. Concentration of Ce3+ and oxygen vacancies in cerium oxide nanoparticles. Chem. Mater. 2006, 18, 5144–5146. 10.1021/cm061580n. [DOI] [Google Scholar]
- Izumi Y.; Hisano K.; Hida T. Acid catalysis of silica-included heteropolyacid in polar reaction media. Appl. Catal., A 1999, 181, 277–282. 10.1016/S0926-860X(98)00399-8. [DOI] [Google Scholar]
- a Devassy B. M.; Halligudi S. B.; Hegde S. G.; Halgeri A. B.; Lefebvre F. 12-Tungstophosphoric acid/zirconia—a highly active stable solid acid—comparison with a tungstated zirconia catalyst. Chem. Commun. 2002, 10, 1074–1075. 10.1039/b200722c. [DOI] [PubMed] [Google Scholar]; b Devassy B. M.; Lefebvre F.; Halligudi S. B. Zirconia-supported 12-tungstophosphoric acid as a solid catalyst for the synthesis of linear alkyl benzenes. J. Catal. 2005, 231, 1–10. 10.1016/j.jcat.2004.09.024. [DOI] [Google Scholar]; c Devassy B. M.; Halligudi S. B. Zirconia-supported heteropoly acids: Characterization and catalytic behavior in liquid-phase veratrolebenzoylation. J. Catal. 2005, 236, 313–323. 10.1016/j.jcat.2005.09.016. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.