Abstract

Branched nanostructures of semiconductors based on one-dimensional heterostructures have many promising applications in optoelectronics, supercapacitors, photocatalysts, etc. Here, we report a novel branched core/shell CdO/ZnO hetero-nanostructure that resembles a Crimson bottlebrush (Callistemon Citrinus) but with intriguing hexagonal symmetry. The nanomaterials were fabricated via an improved one-step chemical vapor deposition method and consist of a CdO wire as the core and ZnO as the shell. With cadmium acting as a catalyst, ZnO nanowires grow as perpendicular branches from the CdO/ZnO one-dimensional core/shell structure. The nanostructures were characterized with X-ray diffraction scanning and transmission electron microscopy. A homogeneous epitaxial growth mechanism has been postulated for the formation of the nanostructure. The materials show a broad and strong absorption ranging from visible to ultraviolet and a better photoelectrocatalytic properties in comparison to pure ZnO or CdO. Our synthetic strategy may open up a new way for controlled preparation of one-dimensional nanomaterials with core/shell heterostructure, which could find potential applications in solar cells and opto-electrochemical water-splitting devices.
1. Introduction
Recently, one-dimensional (1D) core/shell hierarchical hetero-nanostructures have been explored for applications in nanoelectronics, optoelectronics, photocatalysis.1−3 Unlike 1D one-component semiconductors, core/shell hierarchical hetero-nanostructures (or nanocomposite structures) could combine the advantages of each component material to achieve better properties synergistically. The core/shell hetero-nanostructures were demonstrated to enhance separation of the charge, reduce the band gap, and increase the light absorption range to the visible region, which cause a better electron–hole pair separation under irradiation resulting in higher photocatalytic activities.4−9 As examples, 1D ZnS/CdS heterostructures offer higher and ultrafast photosensitivity in ultraviolet/visible photodetectors.10 Branched ZnS/ZnO heterostructure nanofilms exhibit broad photoresponses in flexible ultraviolet photodetectors.11 CdS/ZnO core/shell nanofibers show excellent visible light photocatalytic activity and stability for hydrogen production.12 ZnO@CdS core/shell heterostructures facilitate the degradation of methylene blue under ultraviolet light irradiation.13
Photoelectrochemical (PEC) water splitting as a class of low-cost, pollution-free renewable energy technologies has been studied extensively.14−16 As one of the most studied photocatalysts, TiO2 has a 3.2 eV band gap, high photocatalytic efficiency, and environment-friendly, stable chemical and physical properties.4,17−19 ZnO has become a potential photocatalyst with a similar band gap compared to TiO2, but it has higher electron mobility, has broader absorption of the solar light, is nontoxic, has lower cost, and is easier to fabricate.20,21 However, the high rate of electron recombination in ZnO and the strong tendency of oxidization under UV irradiation lead to oxidative deactivation, affecting its photoelectric catalytic efficiency and stability.22,23 Although many efforts have been made, such as doping, loading precious metals, and combining with other semiconductors to improve the efficiency of photogenerated carriers and extend the spectral response range, few of them were found effective.24−26 Cadmium oxide nanowire (NW), as an important semiconductor with a direct band gap of 2.3 eV (also reported as an indirect semiconductor with a band gap of 1.36 eV) and a promising material in application of sensing and flat-panel displays,27,28 may provide a possible approach to resolve the above-mentioned problems of ZnO. With well-developed bulk and epitaxial growth processes, it can be readily prepared and usually used as a photoanode material in solar cells, sensors, optoelectronics, and photovoltaic-based PEC water-splitting devices.
Most hetero (or composite) structures are prepared in two steps. As examples, 1D ZnS/CdS heterostructures10 or ordered CdS micro/nanostructures on CdSe nanostructures31 were fabricated via a two-step thermal evaporation method; hierarchical assembly of ZnO nanostructures on SnO2 backbone nanowires8 or branched ZnS/ZnO heterostructure11 were prepared by a thermal evaporation process followed by hydrothermal growth; one-dimensional CdS/ZnO core/shell nanofibers12 were made by spinneret electrospinning. So far, there are very few report on the fabrication of CdO nanowires, especially none on the branched CdO/ZnO core/shell heterojunction nanowires, by simple chemical vapour deposition (CVD) methods.29,30
Here, we report for the first time a novel branched CdO/ZnO heterogeneous core/shell nanostructure by an improved CVD method. Unlike other 2-step synthetic methods for core/shell heterostructures, herein we have developed an improved CVD method by the source-moving technique to achieve controllable growth of core/shell heterostructure in one step, which is superior in terms of low cost, high efficiency, easy fabrication, and structural controllability of the samples of uniformed morphology in a large scale.
Although there are many reports related to the core/shell structures, in this work, the CdO/ZnO core/shell structure is reported for the first time and the epitaxial growth of 6-fold symmetrical branches was first observed. A plausible growth mechanism was proposed. The nanomaterials were characterized by scanning electron microscopy (SEM), focused ion beam (FIB)-SEM, transmission electron microscopy (TEM), and X-ray diffraction (XRD). The results indicate that these nanomaterials have a very good crystallinity, branched and core/shell structures. The absorption spectra show that the branched CdO/ZnO heterostructures have a stronger and broader absorption band from UV to visible and to near-infrared light in comparison with those of pure zinc oxide and pure cadmium oxide. Moreover, the CdO/ZnO composite material has a significant increase in current response, and its PEC performance has been enhanced in both the dark and light conditions. The hierarchical and the core/shell structures might not only enhance the capture of light but also provide more surface active sites due to the large specific surface area. Compared with pure zinc oxide, the heterogeneous structure may increase the separation of effective charges, suppress the recombination of electron–hole pairs, and, therefore, hence improve the activity of photocatalyst, as further verified by the impedance spectroscopy.
2. Experimental Section
2.1. Method
An improved CVD route32 was used to grow 1D branched CdO/ZnO heterostructures, where a moving source method was applied in the typical process, as shown in Supporting Information Figure S1. For a typical procedure, a 7:1 weight mixture of ZnO (Macklin, 99.9%) and carbon (Tianjin Guangfu, Spectrography grade) powders were mixed and placed in an alumina boat in the upstream of the tube. Meanwhile, another alumina boat loaded with CdO powder (Aladdin, 99.99%) was placed at the center of the furnace in the tube. The substrate, a silicon wafer coated with a 5 nm thick gold film, was placed 10 cm away from the center of the furnace at the downstream side. In the growth process, we used a quartz rod driven by a step motor through magnetic force to push the boats into the heating zone, and the pressure in the quartz tube was maintained at vacuum atmospheric pressure. After purging the system with 99.99% N2 at 120 standard cubic centimeter per minute (sccm) for 5 min, the furnace was heated to 1080 °C over 50 min and held for 20 min with a N2 (N2 also acts as carrier gas) flow rate of 90 sccm. Then, the ZnO powder was moved to the center of the heating zone slowly within 5 min and the temperature of the furnace was reduced to 1000 °C. After 30 min of growth at 1000 °C, the furnace was naturally cooled down to room temperature. The branched CdO/ZnO heterostructures as composite nanowires (NWs) were grown on the substrate on a large scale. As control experiments, pure CdO nanowires and pure ZnO nanowires were also prepared via the same procedures (please see Supporting Information Figure S2).
2.2. Material Characterizations
The as-grown nanomaterials were characterized utilizing field emission scanning electron microscope (FE-SEM, Zeiss sigma-HD), microscope X-ray diffraction (XRD) (Rigaku D/Max 2500), and a transmission electron microscope (TEM, Titan G2 60-300 with image corrector) equipped with energy-dispersive X-ray spectroscope. The UV–vis absorption spectra were recorded on a UV spectrometer (UV-2600).
2.3. PEC Measurements
All the PEC measurements were performed following the literature procedures6 on an electrochemical workstation (CHI660E 412841) in a three-electrode system, using a prepared photoelectric anode as the working electrode, Pt as the counter electrode, and the standard Ag/AgCl electrode as the reference electrode. The photoresponse of the prepared photoelectrodes was measured under an irradiation source from a 300 W Xe lamp with the filter VisREF (350−-780 nm). The intensity of the light source is estimate about 150 mW/cm2, and 0.1 M KOH aqueous solution acts as the electrolyte. Photoelectric responses of photoanode were measured at an applied potential of −1.15 V vs Ag/AgCl under illumination for light on–off cycles. The electrochemical impedance spectroscopy (EIS) is measured in the frequency range of 0.01–10 kHz.
3. Results and Discussion
3.1. Morphology and Structure Characterization
A typical scanning electron microscopy (SEM) image of the pure CdO nanowires (NWs) grown by our improved method is shown in Figure 1a, revealing that the nanowires have a width of 1–1.5 μm and a length of over 50 μm. We observed that there were no discernable catalyst particles at the tip of the nanowires, which may be due to the fact that the catalyst particles were melted or dropped at high temperatures. An enlarged view of the CdO nanowires given as an inset of Figure 1a shows that the nanowires have a cubic crystal structure. The XRD pattern of the CdO nanowires are depicted in Figure 1b. The diffraction peaks can be indexed to crystalline CdO phase, showing that the product matches well with the cubic phase of CdO with a cell constant a = 0.4695 nm (JCPDS card No. 05-0640). No other peaks are detected in the XRD pattern, indicating high purity of the product. Figure 1c,d shows the typical TEM images of the CdO nanowires and the corresponding selected area electron diffraction (SAED) pattern along the [111] zone axis, respectively, which further identify the CdO structure as cubic crystals. These results are consistent with those in the literature33,34 and validate that our improved method can produce crystalline CdO nano/microwires.
Figure 1.
(a) Typical SEM image of numerous CdO NWs. The inset is an enlarged view of the top and side of the as-grown NWs. (b) XRD pattern of the CdO NWs. (c, d) TEM image together with the SAED pattern of the CdO NW.
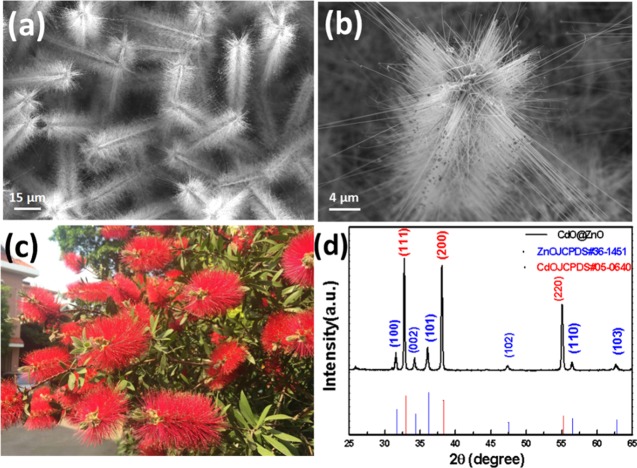
The typical low- and high-magnification SEM images of the branched CdO/ZnO heterostructures are displayed in Figure 2. In Figure 2a, the low-magnification image shows that the nanostructures we synthesized are uniform in a large scale (see Supporting Information Figure S4a), revealing that the Crimson bottlebrush-like (shown in Figure 2c) branched architectures with a width of about 1.5–2 μm and a length of over 50 μm. It is interesting to note that the branched nanostructures have a 6-fold symmetry when observed at a high magnification, as shown Figure 2b, and branches consist of bundles of individual wires with a length of about 9 μm. It is quite apparent that the branches are oriented at an angle of 90° to the backbone nanostructure. Figure 2d is a typical XRD pattern of this branched CdO/ZnO heterostructures. It shows the biphase diffraction pattern of CdO (a = b = 4.695 Å, JCPDS card No. 05-0640) and ZnO (a = 3.25 Å and c = 5.21 Å, JCPDS Card No. 36-1451) crystals. Cadmium oxide’s diffraction peaks are stronger than those of zinc oxide’s, suggesting that in this branched heterostructure, the trunk (i.e., the backbone structure) is cadmium oxide wire, whereas the branches are the zinc oxide nanowires. No new peaks or peak shift are detected in the XRD, indicating that no significant cross-reaction between ZnO and CdO occurred and heterostructures with high phase purity were formed. The relatively strong (111) and (101) diffraction peaks of CdO and ZnO revealed that the growth of heterostructures may be preferentially oriented along [111] and [0001] directions, respectively.
Figure 2.
(a) Low-magnification SEM images of the branched CdO/ZnO heterostructures. (b) High-magnification SEM images of the single branched CdO/ZnO heterostructures. (c) A photograph of a Crimson bottlebrush (photograph courtesy of Qiang Wan. Copyright 2016). (d) XRD pattern of the branched CdO/ZnO heterostructures.
As shown in Figure 2b, there are many particles on the tips of branched nanowires. Figure 3a–d shows an enlarged view of the tip particles with the corresponding element distribution mapping. It can be seen that the tip particles are derived from cadmium (Figure 3d), whereas the branch wires are rich in zinc, providing the basis for our postulation of the growth mechanism of the branches. Figure 3e–h shows the FIB-SEM image of the 1D branched CdO/ZnO heterostructures with the corresponding element distribution mapping. The trunk is a Cd-rich core, whereas the outer layer is a Zn-rich shell. From the bright and dark contrast of its cross section, especially viewed from its top (Figure 3e), a core/shell structure is clearly visible (more details show in Supporting Information Figure S5). Based on these observations, we conclude that the cadmium oxide wires form the core and the zinc oxide wires form the shell in the CdO/ZnO heterostructures. And, the ZnO branches as shell are likely to grow by a homogeneous epitaxial mechanism from the CdO core. These conclusions are further supported by the TEM results as described below.
Figure 3.
(a) SEM image of enlarged view of the top particles in the 1D branched CdO/ZnO heterostructures. (b–d) Elemental distribution of Zn, O, and Cd elements in the top particles. (e) The FIB-SEM image of the 1D branched CdO/ZnO heterostructures. (f–h) Elemental distribution of Zn, O, and Cd elements in the trunk of CdO/ZnO heterostructures.
The branched CdO/ZnO heterostructures were investigated by means of high-resolution transmission electron microscopy (HRTEM). A typical TEM image of an individual CdO/ZnO branched nanostructure is shown in Figure 4a, which confirms that the branches are oriented at an angle of 90° to the backbone nanostructure and the diameters of the zinc oxide branches (about 200 nm) and cadmium oxide trunk (about 2 μm) are relatively uniform. It is noteworthy that in the process of transferring the samples, a certain amount of branches have fractured and broken off from the backbone. Figure 4b is an enlarged image of the branches, where we can see that the zinc oxide branches have a width of 180–210 nm and a length of more than 3 μm. The HRTEM image from the white framed area in Figure 4b is shown in Figure 4c. The crystals exhibit clear lattice fringes with d-spacing of 0.52 and 0.28 nm, which agreed well with the lattice spacing of the (001) planes and the (100) planes of the wurtzite ZnO crystal structure. The inset shows the fast Fourier transformation (FFT) pattern, further proving the branches have a single crystal structure and the preferred wire growth direction is along the [0001] orientation. Figure 4d is the corresponding SAED patterns along the [1210] zone axis, further identifying ZnO with a hexagonal wurtzite structure.
Figure 4.
(a, b) TEM images of 1D branched core/shell CdO/ZnO heterostructures. (c) HRTEM image of ZnO nanowires. (d) The corresponding SAED pattern. The inset of (d) is the FFT pattern. (e) The high-angle annular dark field (HAADF) map of 1D branched core/shell CdO/ZnO heterostructures. (f–h) Elemental distribution of Cd, O, and Zn elements in 1D branched core/shell CdO/ZnO heterostructures. The inset of (f) is the combined elemental distribution mapping of Cd and Zn elements.
To further analyze the heterojunction component information, the diagram in Figure 4e–h shows the distribution of the corresponding elements, where Figure 4e is the high-angle annular dark field (HAADF) map. Through the central and peripheral contrast of light and dark in the figure, we could identify the heterogeneity of the growth with core/shell structures. And, Figure 4f–h reveal the distribution of the constituting elements O, Zn, and Cd, respectively. Oxygen is distributed uniformly in the entire space and cadmium and zinc are uniformly distributed in the trunk and branches, respectively. These results further support the information that the trunk/core consisting of cadmium oxide and zinc oxide undergoes epitaxial growth of branches as shell and finally forms the heterogeneous core/shell branched structure, as is clearly identifiable in the inset in Figure 4f.
3.2. Postulated Growth Mechanism
This 1D branched core/shell CdO/ZnO heterostructured nanomaterials were fabricated by an improved CVD technique. The growth process of this novel structure presented here can be roughly described in three separate stages, as illustrated in Figure 5. The first stage is the growth of CdO nanowires, which act as subsequent phase nucleation sites of growths, typically following the vapor–liquid–solid (VLS) mechanism.35−37 When the furnace increased to the setting temperature, the CdO source (Aladdin, 99.99%) was started to evaporate into vapor phase at the center of the tube and the hemispherical gold liquid located downstream was formed on the Si substrate, which was coated with the gold film in advance. Then, the CdO vapor was transported by carrier gas and reacted with Au, which acts as a catalyst to form alloy droplets. As the droplets became supersaturated, crystalline CdO nanowires precipitated with a liquid catalyst at the tip. Subsequently, the growth of nanowires began. We observed that there were no discernable catalyst particles on the tips of the CdO nanowires, which might be attributed to the Au catalyst particles melting or falling off at high temperatures.38 Here, the Au particles play a very important role in the growth of the CdO nanowires.39 The second stage of the growth was the formation of ZnO shells.40 With the mixture of ZnO and carbon spectrography powders pushed into the center of the tube, the following reactions could take place41
When the concentrations of the Zn and O vapors increased rapidly and reached a supersaturation state during the chemical reaction process, it was easy to form a nucleation site on the CdO nanowires sidewall with a high crystal lattice energy. As the vapor was transported downstream by carrier gas, the CdO nanowires acted as a collector and the ZnO vapor deposited on it, leading to the formation of the core/shell structure. The shell of the ZnO nanowires has a typical hexagonal structure, which was determined by its six equivalent crystal planes, i.e., ±(101̅0), ±(1̅100), and ±(011̅0).
Figure 5.
Growth process of the 1D branched core/shell CdO/ZnO heterostructures.
The third stage is the homoepitaxial growth of the branches. When the vapor concentration decreased to a certain extent, the dendritic ZnO nanowires start to grow from the ZnO shell’s surface, where the Cd liquid droplets, which came from the reduction of cadmium oxide by carbon monoxide, act as catalysts, as manifested by the SEM results in Figure 3. In addition, the screw dislocation on the shell may act as a factor in the growth of branches, which is a subject for further investigation.42,43 It should be emphasized that this is a preliminary proposal for the mechanism of the structure formation and that many details remain unresolved. We are going to work on collecting more data and refining our proposal.
3.3. Diffuse Reflectance Properties
The optical reflectance properties of the CdO nanowires, the ZnO nanowires, and the CdO/ZnO nanostructures were investigated by UV–vis diffuse reflectance spectrum (DRS). As shown in Figure 6, the ZnO nanowires exhibit a characteristic absorption peak near 380 nm, which agrees well with the band gap of 3.37 eV of ZnO. The CdO nanowires had a strong absorption below 550 nm, which should be ascribed to the excitonic absorption feature of CdO.34 It is noted that the absorption peaks at about 350 and 520 nm are not sharp, indicating that the absorption bands might have originated from both direct and indirect transitions, which is a characteristic of the CdO material.44 The UV–vis DRS of the CdO/ZnO nanostructures shows a strong absorption in visible light and tailing into the near-infrared range as compared with the ZnO nanowires. The broad band in the vicinity of 500 nm may be attributed to CdO absorption. Thus, branched CdO/ZnO core/shell heterostructures have quite strong and broad absorption bands ranging from visible to ultraviolet light, suggesting that the photocatalytic efficiency of the CdO/ZnO nanostructures under solar irradiation could have a better performance.
Figure 6.

UV–vis diffuse reflectance spectra of CdO nanowires, ZnO nanowires, and CdO/ZnO nanostructures.
3.4. Photoelectrochemical Characterization
The photoelectrochemical (PEC) performance of the Si substrate, the ZnO nanowires, and the CdO/ZnO nanostructures was evaluated by measuring the photocurrent response, as shown in Figure 7. Figure 7a is the current density–voltage curves recorded in dark and under illumination by linear sweep voltammetry (LSV) at the scan rate of 5 mV/s from −0.9 to −1.4 V (vs Ag/AgCl). The light source was a 300 W xenon lamp with 350–780 nm wavelength, 150 mW/cm2 intensity, and 15 cm distance between the light and the samples. The current density of the CdO/ZnO hetero-nanostructures was up to 27 mA/cm2 in dark, which is a significant enhancement compared to pure ZnO.
Figure 7.
(a) LSV collected at a scan rate of 5 mV/s in the dark and under illumination for the samples. (b) Chronoamperometric (I–t) curves at an applied potential of −1.15 V vs Ag/AgCl under illumination with 70 s light on–off cycles.
Upon light exposure, all the samples show an increase in photocurrent to various extents. In particular, the photocurrent of the CdO/ZnO hetero-nanostructures reached a maximum of 35 mA/cm2 under illumination, a substantial increase over pure zinc oxide. This could be attributed to the hierarchical structure of the heterogeneous nanowires that might have the high separation efficiency of the electron–hole pairs, as well as the strong absorption of light. The chronoamperometric (I–t) experiments were carried out at an applied potential of −1.15 V vs Ag/AgCl under illumination with 70 s light on–off cycles to further assess the stability of the photoelectrodes. As shown in Figure 7b, the photocurrent generation was triggered instantaneously upon illumination and reached a steady state in each cycle (for more details, see Supporting Information Figure S6).
To further investigate the PEC activity of the semiconductor materials in which the bulk conductivity as well as the interfacial charge transfer were critical factors, EIS measurements were performed under illumination in the frequency range of 10 000–0.01 Hz with an alternating current amplitude of 5 mV.45 In the Nyquist spectra (Figure 8), the semicircle at high frequencies represent the charge transfer process, and its radius represents the size of the electrical transmission resistance. In general, the smaller radius indicates a faster electron transport rate and a more efficient separation of the photogenerated electrons and holes.24 As a comparison, the EIS data for silicon substrate and ZnO nanowires were also charactered (Figure 8). The CdO/ZnO photoelectrode shows the smallest semicircle portion, indicating that it has the smallest charge transfer resistance and the highest charge separation efficiency. This could be attributed to the heterogeneous structures between ZnO and CdO, which could improve the charge separation efficiency.
Figure 8.

Comparative Nyquist plots of the EIS data for silicon, ZnO nanowires, and CdO/ZnO nanostructures.
4. Conclusions
We have successfully synthesized a novel 1D branched core/shell CdO/ZnO heterostructure with high crystallinity by an improved chemical vapor deposition method. This novel nanomaterial has a Crimson bottlebrush-like structure in which the CdO nanowire forms the core/trunk, whereas the hexagonal ZnO grows around the CdO trunk to form the shell. On the shell, the ZnO nanowires were homogeneous epitaxial grown perpendicular to the it via VLS mechanism while the cadmium acts as catalyst. So that the 6-fold symmetrical branches formed. To the best of our knowledge, this is the first report of cadmium as a catalyst. Such a nanostructure is quite uniform in a large scale throughout the observed surface of the silicon substrate. The structure has been thoroughly characterized and its formation mechanism has been proposed. Because of the heterogeneous composite nanostructure, it exhibits interesting optical and photoelectrochemical properties. It has much stronger and broader light absorption, ranging from UV to visible and near IR, than pure CdO or ZnO nanowires. The photoelectrochemical activity and the electrochemical impedance spectral measurements show that the charge collection efficiency of the 1D hierarchical core/shell nanostructure has also been improved significantly over that of ZnO nanowires. The heterogeneous branched core/shell nanostructure might prevent or inhibit the recombination of the electron–hole pairs to a certain extent and, hence, improve the effective charge transfer so that it could be promising for applications as a more effective photocatalyst. Further investigation to quantify these optical and photoelectrochemical effects and elucidate the mechanism of the heterostructure formation is in progress. Moreover, such novel nanostructured materials would also find applications in a variety of fields, such as field emission, photovoltaics, water decomposition, supercapacitors, fuel cells, high-strength and multifunctional nanocomposites, etc.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (51302078, 61501039), the Beijing Natural Science Foundation (2162017), the Beijing Municipal Education Commission (KM201710015005), the Elite Program of BIGC (04190117004/010), the Initial Funding for the Doctoral Program of BIGC (27170115005/040), and the Beijing Collaborative Innovation for Green Printing and Publication (04190117029/002).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b00457.
Growth details of the 1D hierarchical core/shell nanostructure and the stability of the CdO/ZnO photoanode (PDF).
Author Contributions
∥ P.S. and R.L. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Gautam U. K.; Fang X. S.; Bando Y.; Zhan J. H.; Golberg D. Synthesis, Structure, and Multiply Enhanced Field-Emission Properties of Branched ZnS Nanotube-In Nanowire Core-Shell Heterostructures. ACS Nano 2008, 2, 1015–1021. 10.1021/nn800013b. [DOI] [PubMed] [Google Scholar]
- Ko S. H.; Lee D.; Kang H. W.; Nam K. H.; Yeo J. Y.; Hong S. J.; Grigoropoulos C. P.; Sung H. J. Nanoforest of Hydrothermally Grown Hierarchical ZnO Nanowires for a High Efficiency Dye-Sensitized Solar Cell. Nano Lett. 2011, 11, 666–671. 10.1021/nl1037962. [DOI] [PubMed] [Google Scholar]
- Mishra Y. K.; Modi G.; Cretu V.; Postica V.; Lupan O.; Reimer T.; Paulowicz I.; Hrkac V.; Benecke W.; Kienle L.; Adelung R. Direct Growth of Freestanding ZnO Tetrapod Networks for Multifunctional Applications in Photocatalysis, UV Photodetection, and Gas Sensing. ACS Appl. Mater. Interfaces 2015, 7, 14303–14316. 10.1021/acsami.5b02816. [DOI] [PubMed] [Google Scholar]
- Qiu Y.; Ouyang F. Fabrication of TiO2 hierarchical architecture assembled by nanowires with anatase/TiO2(B) phase-junctions for efficient photocatalytic hydrogen production. Appl. Surf. Sci. 2017, 403, 691–698. 10.1016/j.apsusc.2017.01.255. [DOI] [Google Scholar]
- Chen H. Y.; Xu Y. F.; Kuang D. B.; Su C. Y. Recent advances in hierarchical macroporous composite structures for photoelectric conversion. Energy Environ. Sci. 2014, 7, 3887–3901. 10.1039/C4EE02213K. [DOI] [Google Scholar]
- Chen W.; Ruan H.; Hu Y.; Li D.; Chen Z.; Xian J.; Chen J.; Fu X.; Shao Y.; Zheng Y. One-step preparation of hollow ZnO core/ZnS shell structures with enhanced photocatalytic properties. CrystEngComm 2012, 14, 6295–6305. 10.1039/c2ce25591j. [DOI] [Google Scholar]
- Lao J. Y.; Wen J. G.; Ren Z. F. Hierarchical ZnO Nanostructures. Nano Lett. 2002, 2, 1287–1291. 10.1021/nl025753t. [DOI] [Google Scholar]
- Cheng C. W.; Liu B.; Yang H. Y.; Zhou W. W.; Sun L.; Chen R.; Yu S. F.; Zhang J. X.; Gong H.; Sun H. D.; Fan H. J. Hierarchical Assembly of ZnO Nanostructures on SnO2 Backbone Nanowires: Low-Temperature Hydrothermal Preparation and Optical Properties. ACS Nano 2009, 3, 3069–3076. 10.1021/nn900848x. [DOI] [PubMed] [Google Scholar]
- Hu L. F.; Yan J.; Liao M. Y.; Xiang H. J.; Gong X. G.; Zhang L. D.; Fang X. S. An Optimized Ultraviolet-A Light Photodetector with WideRange Photoresponse Based on ZnS/ZnO Biaxial Nanobelt. Adv. Mater. 2012, 24, 2305–2309. 10.1002/adma.201200512. [DOI] [PubMed] [Google Scholar]
- Lou Z.; Li L. D.; Shen G. Z. Ultraviolet/visible photodetectors with ultrafast, high photosensitivity based on 1D ZnS/CdS heterostructures. Nanoscale 2016, 8, 5219–5225. 10.1039/C5NR08792A. [DOI] [PubMed] [Google Scholar]
- Tian W.; Zhang C.; Zhai T. Y.; Li S.-L.; Wang X.; Liu J. W.; Jie X.; Liu D. Q.; Liao M. Y.; Koide Y.; Golberg D.; Bando Y. Flexible Ultraviolet Photodetectors with Broad Photoresponse Based on Branched ZnS-ZnO Heterostructure Nanofilms. Adv. Mater. 2014, 26, 3088–3093. 10.1002/adma.201305457. [DOI] [PubMed] [Google Scholar]
- Yang G.; Yan W.; Zhang Q.; Shen S. H.; Ding S. J. One-dimensional CdS/ZnO core/shell nanofibers via single-spinneret electrospinning: tunable morphology and efficient photocatalytic hydrogen production. Nanoscale 2013, 5, 12432–12439. 10.1039/c3nr03462c. [DOI] [PubMed] [Google Scholar]
- Ding M.; Yao N. N.; Wang C. G.; Huang J. Z.; Shao M. H.; Zhang S. W.; Li P.; Deng X. L.; Xu X. J. ZnO@CdS Core-Shell Heterostructures: Fabrication, Enhanced Photocatalytic, and Photoelectrochemical Performance. Nanoscale Res. Lett. 2016, 11, 205. 10.1186/s11671-016-1432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishima A.; Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- Tachibana Y.; Vayssieres L.; Durrant J. R. Artificial photosynthesis for solar water-splitting. Nat. Photonics 2012, 6, 511–518. 10.1038/nphoton.2012.175. [DOI] [Google Scholar]
- Kim D.; Sakimoto K. K.; Hong D.; Yang P. Artificial photosynthesis for sustainable fuel and chemical production. Angew. Chem. Int. Ed. 2015, 54, 3259–3266. 10.1002/anie.201409116. [DOI] [PubMed] [Google Scholar]
- Schneider J.; Matsuoka M.; Takeuchi M.; Zhang J.; Horiuchi Y.; Anpo M.; Bahnemann D. W. Understanding TiO2 photocatalysis: mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. 10.1021/cr5001892. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Ma L.; Zhao Q.; Li Z.; Xu X. Mophology-modulations of TiO2 nanostructures for enhanced photocatalytic performance. Appl. Surf. Sci. 2015, 332, 224–228. 10.1016/j.apsusc.2015.01.052. [DOI] [Google Scholar]
- Wang R.; Jiang G.; Ding Y.; Wang Y.; Sun X.; Wang X.; Chen W. Photocatalytic activity of heterostructures based on TiO2 and halloysite nanotubes. ACS Appl. Mater. Interfaces 2011, 3, 4154–4158. 10.1021/am201020q. [DOI] [PubMed] [Google Scholar]
- Wang X.; Summers C. J.; Wang Z. L. Large-scale hexagonal-patterned growth of aligned ZnO nanorods for nano-optoelectronics and nanosensor arrays. Nano Lett. 2004, 4, 423–426. 10.1021/nl035102c. [DOI] [PubMed] [Google Scholar]
- Son D.-Y.; Im J.-H.; Kim H.-S.; Park N.-G. 11% efficient perovskite solar cell based on ZnO nanorods: an effective charge collection system. J. Phys. Chem. C 2014, 118, 16567–16573. 10.1021/jp412407j. [DOI] [Google Scholar]
- Xu F.; Yuan Y.; Han H.; Wu D.; Gao Z.; Jiang K. Synthesis of ZnO/CdS hierarchical heterostructure with enhanced photocatalytic efficiency under nature sunlight. CrystEngComm 2012, 14, 3615–3622. 10.1039/c2ce06267d. [DOI] [Google Scholar]
- Yu Z. B.; Xie Y. P.; Liu G.; Lu G. Q.; Ma X. L.; Cheng H. M. Self-assembled CdS/Au/ZnO heterostructure induced by surface polar charges for efficient photocatalytic hydrogen evolution. J. Mater. Chem. A 2013, 1, 2773–2776. 10.1039/c3ta01476b. [DOI] [Google Scholar]
- Khan A.; Ahmed M. I.; Adam A.; Azad A.-M.; Qamar M. A novel fabrication methodology for sulfur doped ZnO nanorods as an active photoanode for improved water oxidation in visible-light regime. Nanotechnology 2017, 28, 055602 10.1088/1361-6528/aa51b6. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Du L.; Yu X.; Tan S.; Cai X.; Yang P.; Gu Y.; Mai W. Significantly enhanced photocatalytic activities and charge separation mechanism of Pd decorated ZnO-graphene oxide nanocomposites. ACS Appl. Mater. Interfaces 2014, 6, 3623–3629. 10.1021/am405872r. [DOI] [PubMed] [Google Scholar]
- Kundu P.; Deshpande P. A.; Madras G.; Ravishankar N. Nanoscale ZnO/CdS heterostructures with engineered interfaces for high photocatalytic activity under solar radiation. J. Mater. Chem. 2011, 21, 4209–4216. 10.1039/c0jm03116j. [DOI] [Google Scholar]
- Ristić M.; Popovic S.; Music S. Formation and properties of Cd (OH)2 and CdO particles. Mater. Lett. 2004, 58, 2494–2499. 10.1016/j.matlet.2004.03.016. [DOI] [Google Scholar]
- Kanjwal M. A.; Barakat N.; Sheikh F. A.; Kim H. Y. Electronic Characterization and Photocatalytic Properties of TiO2/CdO Electrospun Nanofibers. J. Mater. Sci. 2010, 45, 1272–1279. 10.1007/s10853-009-4078-3. [DOI] [Google Scholar]
- Saravanan R.; Shankar H.; Prakash T.; Narayanan V.; Stephen A. ZnO/CdO composite nanorods for photocatalytic degradation of methylene blue under visible light. Mater. Chem. Phys. 2011, 125, 277–280. 10.1016/j.matchemphys.2010.09.030. [DOI] [Google Scholar]
- Karami H. Investigation of sol-gel Synthesized CdO-ZnO Nanocomposite for CO Gas Sensing. Int. J. Electrochem. Sci. 2010, 5, 720–730. [Google Scholar]
- Dai G.; Peng Z. W.; Zhang Q. L.; Zhou W. C.; Xia M. X.; Li H. X.; Pan A. L.; Wan Q.; Zou B. S. Ordered CdS micro/nanostructures on CdSe nanostructures. Nanotechnology 2009, 20, 125601 10.1088/0957-4484/20/12/125601. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Liu R. P.; Ma L.; Li D.; Yang Y. K.; Dai G. Z.; Wan Q. Fabrication of GaInPSb quaternary alloy nanowires and its room temperature electrical properties. Appl. Phys. A 2017, 123, 6. 10.1007/s00339-016-0590-x. [DOI] [Google Scholar]
- Kuo T.-J.; Huang M. H. Gold-Catalyzed Low-Temperature Growth of Cadmium Oxide Nanowires by Vapor Transport. J. Phys. Chem. B 2006, 110, 13717–13721. 10.1021/jp062854x. [DOI] [PubMed] [Google Scholar]
- Kumar S.; Ojha A. K. Synthesis, characterizations and antimicrobial activities of well dispersed ultra-long CdO nanowires. AIP Adv. 2013, 3, 052109 10.1063/1.4804930. [DOI] [Google Scholar]
- Wagner R. S.; Ellis W. C. Synthesis, characterizations and antimicrobial activities of well dispersed ultra-long CdO nanowires. Appl. Phys. Lett. 1964, 4, 89–90. 10.1063/1.1753975. [DOI] [Google Scholar]
- Huang M. H.; Mao S.; Feick H.; Yan H. Q.; Wu Y. Y.; Kind H.; Weber E.; Russo R.; Yang P. D. Room-Temperature Ultraviolet Nanowire Nanolasers. Science 2001, 292, 1897–1899. 10.1126/science.1060367. [DOI] [PubMed] [Google Scholar]
- Dai G. Z.; Chen Y.; Wan Q.; Zhang Q. L.; Pan A. L.; Zou B. S. Fabrication and optical waveguide of Sn-catalyzed CdSe microstructures. Solid State Commun. 2013, 167, 31–35. 10.1016/j.ssc.2013.05.013. [DOI] [Google Scholar]
- Dai G. Z.; Gou G. Y.; Wu Z. M.; Chen Y.; Li H. J.; Wan Q.; Zou B. S. Fabrication and micro-photoluminescence property of CdSe/CdS core/shell nanowires. Appl. Phys. A 2015, 119, 343–349. 10.1007/s00339-014-8973-3. [DOI] [Google Scholar]
- Wagner R. S.; Ellis W. C. Vapor-Liquid-Solid mechanism of single crystal growth. Appl. Phys. Lett. 1964, 4, 89–90. 10.1063/1.1753975. [DOI] [Google Scholar]
- Chen J.; Wu X.-J.; Gong Y.; Zhu Y. H.; Yang Z. Z.; Li B.; Lu Q. P.; Yu Y. F.; Han S. K.; Zhang Z. C.; Zong Y.; Han Y.; Gu L.; Zhang H. Edge Epitaxy of Two-Dimensional MoSe2 and MoS2 Nanosheets on One-Dimensional Nanowires. J. Am. Chem. Soc. 2017, 139, 8653–8660. 10.1021/jacs.7b03752. [DOI] [PubMed] [Google Scholar]
- Farnsworth M.; Kline C.. Zinc Chemicals, Their Properties and Applications; International Lead and Zinc Research Organization: New York, 1968. [Google Scholar]
- Jia Z.; Hailin P.; Candace K. C.; Konrad J.; Xiao Feng Z.; Yi C. Hyperbranched Lead Selenide Nanowire Networks. Nano Lett. 2007, 7, 1095–1099. 10.1021/nl0700393. [DOI] [PubMed] [Google Scholar]
- Jia Z.; Hailin P.; Marshall A. F.; Barnett D. M.; Nix W. D.; Yi C. Formation of chiral branched nanowires by the Eshelby Twist. Nat. Nanotech. 2008, 3, 477–481. 10.1038/nnano.2008.179. [DOI] [PubMed] [Google Scholar]
- Zou S. Y.; Zhou W. C.; Liu R. B.; Zou B. S. Cavity-Enhanced Microphotoluminescence in a Core–Shell n–p CdS/CdO Micrometer Wire and Its Efficient Surface Photovoltage Responses in the Whole Visible Range. J. Phys. Chem. C 2017, 121, 14349–14358. 10.1021/acs.jpcc.7b04053. [DOI] [Google Scholar]
- Jiang J.; Zhang X.; Sun P.; Zhang L. ZnO/BiOI heterostructures: photoinduced charge-transfer property and enhanced visible-light photocatalytic activity. J. Phys. Chem. C 2011, 115, 20555–20564. 10.1021/jp205925z. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.