Abstract
Background
Significant coronary artery disease has a well‐known association with long‐term adverse cardiovascular events. In this study, we aimed to evaluate its association with long‐term major adverse clinical events (MACE) up to 5 years in patients who presented with chest pain without significant coronary artery disease.
Methods and Results
A total of 5890 subjects with chest pain without significant coronary artery disease were prospectively enrolled in this study. The mean follow‐up duration was 3.4 years. Multivariable Cox proportional hazards regression analysis was performed for assessing the independent risk factors for MACE or sustained angina pectoris. MACE was defined as the composite of total death, myocardial infarction, coronary revascularization, stroke, and hospitalization because of heart failure. Ninety‐one (2.2%) patients developed MACE, and 309 (8.1%) patients developed sustained angina pectoris, both within 5 years. In multivariable Cox proportional hazards regression analysis, the risk of MACE was significantly associated with age (per 5 years; hazard ratio [HR], 1.44; 95% CI, 1.30–1.60) and insignificant coronary stenosis (30%–70%; HR, 2.03; 95% CI; 1.28–3.21). The risk of sustained angina pectoris was significantly associated with age (per 5 years; HR, 1.05; 95% CI, 1.01–1.11), dyslipidemia (HR, 1.34; 95% CI, 1.06–1.70), insignificant coronary stenosis (HR, 2.54; 95% CI, 1.94–3.31), coronary artery spasm (HR, 1.42; 95% CI, 1.11–1.80), and myocardial bridge (HR, 1.37; 95% CI, 1.04–1.81).
Conclusions
In patients without significant CAD, aging and insignificant coronary stenosis have a strong association with future long‐term MACE. Also, aging, dyslipidemia, insignificant coronary stenosis, coronary artery spasm, and myocardial bridge are strongly associated with future angina pectoris.
Keywords: clinical events, coronary artery dissection, coronary angiography, risk factor, risk assessment, vasospasm
Subject Categories: Cardiovascular Disease, Epidemiology, Risk Factors, Coronary Artery Disease, Atherosclerosis
Clinical Perspective
What Is New?
Among patients with chest pain without significant coronary artery disease, coronary artery spasm (CAS), myocardial bridge (MB), and/or insignificant coronary stenosis (ICS) have been frequently found.
From a long‐term clinical evaluation of patients without coronary artery disease, aging and ICS were strongly associated with major adverse cardiac events. Further, aging, dyslipidemia, ICS, CAS, and MB were strongly associated with sustained angina pectoris.
Additionally, during the 5‐year follow‐up period, a minority of enrolled patients experienced stroke (0.6%) and heart failure (0.8%).
What Are the Clinical Implications?
When clinicians are evaluating the cause of chest pain, it may be judged to be cardiovascular if the physician suspects the presence of CAS, MB, and ICS despite absence of significant coronary artery disease.
In particular, CAS, MB, and ICS may exist independently but could also appear in combination with one another.
The combination of CAS, MB, and/or ICS was associated with poor long‐term clinical outcomes compared with single factors. The presence of ICS was the strongest independent predictor for major adverse cardiac events and sustained angina pectoris; therefore, patients with chest pain and ICS should be carefully treated and need close clinical follow‐up, even in the absence of significant coronary artery disease.
Chest pain is a major symptom of ischemic heart disease such as angina pectoris (AP) and acute coronary syndrome (ACS); therefore, it is important to identify and diagnose the causes of pain in these patients.1, 2 The main mechanism of AP is the imbalance between oxygen demand and supply in the myocardium. Obstructive coronary stenosis, coronary artery spasm (CAS), and myocardial bridge (MB) are well‐known causes of myocardial ischemia.1, 2, 3, 4, 5, 6 CAS is a well‐known endothelial dysfunction, and MB is substantially implicated in a high incidence of CAS; thus, both MB and CAS are major causes of vasospastic angina.2, 3, 4 Therefore, if the cause of chest pain is judged to be cardiovascular, clinicians usually evaluate coronary arteries with electrocardiography, stress test, cardiac computed tomography scans, and coronary angiography, (CAG) including the intracoronary acetylcholine (ACH) or ergonovine provocation test.2 Obstructive coronary stenosis requires active treatment with mechanical revascularization and drug intervention, as it is known to be closely related to poor prognoses such as ACS, myocardial infarction (MI), and death.1 However, if no significant coronary lesion is seen on CAG despite chest pain, the scope of determining the prognosis and its association are limited.
Materials and Methods
The design of this registry has been introduced before.3, 4, 6, 7, 8 In brief, it is a single‐center, prospective, all‐comers registry designed to reflect the “real‐world” practice since 2004. Data were collected by a trained study coordinator using a standardized case report form. Standardized definitions for all patient‐related variables and clinical diagnoses were used. The participants or their legal guardians were given a thorough literal and verbal explanation of the study procedures before they provided written consent to participate in the study. The Institutional Review Board (IRB) of Korea University Guro Hospital approved all the procedures where the patients had provided consent. The authors of this paper have certified that the information contained herein is true and correct as reflected in the records of the IRB (#KUGH10045). The Korea University Guro Hospital IRB specifically approved this entire study.
A total of 10 177 subjects with typical or atypical chest pain who underwent CAG at the cardiovascular center of Korea University Guro Hospital, Seoul, Korea between November 2004 and May 2014 were enrolled for this study. Among these, 5890 subjects with typical or atypical chest pain and without significant coronary artery stenosis (defined as having a stenosis diameter of <70%, as seen on the quantitative coronary angiography) underwent an intracoronary ACH provocation test (Figure 1). Patients were excluded if they had any of the following conditions: coronary artery bypass graft, prior percutaneous coronary intervention, stroke, advanced heart failure (HF; New York Heart Association class III or IV) or serum creatinine >2 mg/dL, because these conditions could be major causes of adverse cardiovascular events and could bias the results.
Figure 1.
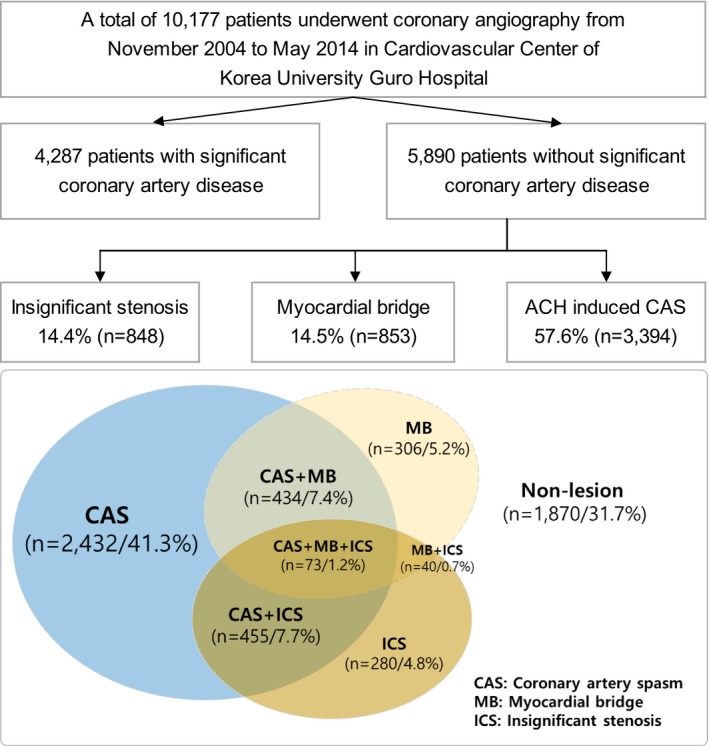
Study flowchart. ACH indicates acetylcholine; CAS, coronary artery spasm; ICS, insignificant coronary stenosis; MB, myocardial bridge.
ACH Provocation Test
The design of the ACH provocation test has been introduced in prior studies.3, 4, 6, 7, 8 Initial investigation for CAG included clinical history taking and noninvasive stress tests such as treadmill test, stress echocardiography, and radionuclide study. CAG was performed to confirm the presence of significant coronary artery disease (CAD). However, CAG was immediately done without functional studies in cases of typical resting ischemic chest pain to confirm vasospastic angina. Vasodilators or vasoconstrictors such as nitrates, calcium channel blockers, beta blockers, nicorandil, and molsidomine were discontinued for at least 72 hours before CAG. CAS induction was tested by an intracoronary injection of ACH immediately after diagnostic angiography by either a transradial or transfemoral approach. ACH was injected by incremental doses of 20 (A1), 50 (A2), and 100 (A3) μg/min into the left coronary artery over a 1‐minute period with 5‐minute intervals up to the maximally tolerated dose under a continuous monitoring with ECG and measuring of blood pressure. Routine provocation test of the right coronary artery was not done because of safety issues regarding the higher prevalence of advanced atrioventricular block, which needs a temporary pacemaker for maintaining adequate ACH infusion rate and cost‐effectiveness for diagnosis and management of significant CAS. Angiography was repeated after each ACH dose until a significant focal or diffuse narrowing of >70% was observed. If significant focal or diffuse vasoconstriction (>70%) of coronary arteries was induced at any dose, ACH infusion was stopped. An intracoronary injection of 0.2 mg of nitroglycerin was administered after completing the ACH provocation test, followed by CAG 2 minutes later. End‐systolic images for each segment of the left coronary artery were chosen according to the corresponding points on the electrocardiographic trace (QRS onset or end of T wave) and analyzed using the proper quantitative comparative analysis system of the catheterization laboratory (FD‐20, Phillips, Amsterdam, The Netherlands). Coronary artery diameters were measured by quantitative comparative analysis before and after the administration of ACH at the site that showed the greatest changes following drug administration. Reference vessel diameters were measured at the proximal and distal portions of each artery. The mean reference vessel diameter was used to assess diameter narrowing by quantitative comparative analysis.
Study Definition
In the present study, significant CAS was defined as >70% luminal narrowing of the artery during the ACH provocation test, regardless of ischemic electrocardiography changes or presence of chest pain.3, 6 MB was defined as having a characteristic phasic systolic compression of the coronary artery, with a decrease of >30% in diameter on the CAG after intracoronary nitroglycerin infusion, which is exclusively performed in the left anterior descending coronary artery, mostly in the anterior‐posterior cranial or right anterior oblique cranial projections.3 Insignificant coronary stenosis (ICS) was defined as having a fixed stenosis of <70% in the epicardial coronary artery; this was further divided into 2 groups according to the severity of ICS (mild, 30%–49%; moderate, 50%–69%).6 Major adverse cardiac events (MACE) were defined as the composite of total death; MI; stroke, hospitalization because of HF; and revascularization, including percutaneous coronary intervention, and coronary artery bypass graft. Deaths were regarded to be attributable to a cardiac cause unless a noncardiac death could be confirmed. Repeat CAG (mostly due to sustained AP) was performed in patients who complained of recurrent angina despite adequate antianginal medication for at least 6 months since the first CAG was performed. In such cases, the physician assumed that the CAS may have progressed or there may be newly developing atherosclerotic CAD.
Statistical Analysis
Differences in continuous variables between the 2 groups were evaluated using the unpaired t test or Mann–Whitney rank test. Data are expressed as means±SDs. For discrete variables, differences are expressed as counts and percentages and were analyzed with the chi‐squared test or Fisher's exact test. Multivariable Cox proportional hazards model analysis was performed for assessing the independent risk factors for MACE or sustained AP. We tested available variables that could be of potential relevance: age, sex, cardiovascular risk factors (hypertension, diabetes mellitus, dyslipidemia, and current smoking), and angiographic and clinical parameters (MB, ICS). We used Kaplan–Meier curves analysis and the log‐rank test to depict the association among groups of various combinations of a nonsignificant coronary lesion and survival free of clinical outcomes. For all analyses, a 2‐sided P<0.05 was considered statistically significant. All data were processed with SPSS (version 20.0, IBM, Armonk, NY).
Study End Points
The primary end point was MACE. The secondary end point was the recurrent angina requiring repeat CAG.
Results
For this study, a total of 5890 subjects with chest pain without significant CAD were ultimately enrolled. The median age of the subjects was 55.7 (range, 18.0–89.7) years. Baseline clinical and angiographic characteristics and 5‐year clinical outcomes are shown in Table 1. All enrolled subjects underwent CAG and the ACH test; 14.4% had ICS, 14.5% had MB, and 57.6% had CAS. CAS, MB, and ICS affect each other, and these combinations are shown in Figure 1. In the 5‐year clinical follow‐up, the incidence of MACE was 2.2%; the all‐cause death was 0.4%, MI was 0.3%, coronary revascularization was 0.4%, stroke was 0.6%, and hospitalization due to HF was 0.8%. The incidence of sustained AP was 8.1%.
Table 1.
Baseline Clinical and Angiographic Characteristics and 5‐Year Clinical Follow‐Up
| Variables | Total (n=5890) | Variables | Total (n=5890) |
|---|---|---|---|
| Sex, male | 2703 (45.9) | Coronary angiography | |
| Age, y | 55.3±12.4 | Insignificant stenosis | |
| Blood pressure, mm Hg | Mild (<30%) | 2834 (48.1) | |
| Systolic | 135±21 | Mild (30–50%) | 481 (8.1) |
| Diastolic | 78±12 | Moderate (50–70%) | 367 (6.2) |
| Heart rate, beats per minute | 71±13 | Myocardial bridge (>30%) | 853 (14.4) |
| Body mass index | 24±3 | CAS (after ACH provocation test) | |
| Patients at risk | Significant CAS (>70%) | 3394 (57.6) | |
| Hypertension | 2694 (45.7) | CAS site | |
| Diabetes mellitus | 928 (15.7) | Left main | 8 (0.2) |
| New‐onset diabetes mellitus | 210 (3.5) | Left arterial descending | 3181 (93.7) |
| Insulin | 100 (1.6) | Left circumflex | 1300 (38.3) |
| Medication | 594 (10.0) | CAS location | |
| Dietary | 71 (1.2) | Mid to distal | 1296 (38.1) |
| Dyslipidemia | 1757 (29.8) | Proximal to distal | 1409 (41.5) |
| History of smoking | 1699 (28.8) | Proximal only | 246 (7.2) |
| Current smoking | 1213 (20.5) | Mid only | 380 (11.1) |
| History of alcohol use | 2050 (34.8) | Distal only | 63 (1.8) |
| Current alcohol use | 1881 (31.9) | Diffuse CAS (>20 mm) | 2913 (85.8) |
| Medication history | Multivessel CAS | 1129 (33.2) | |
| Calcium channel blockers | 2570 (43.6) | ECG change | 255 (4.3) |
| Diltiazem | 315 (5.3) | Clinical follow‐up at 5 years | |
| Nitrate | 279 (4.7) | Total death | 16 (0.4) |
| Trimetazidine | 176 (2.9) | Cardiac death | 6 (0.1) |
| Molsidomine | 24 (0.4) | MI | 12 (0.3) |
| Nicorandil | 143 (2.4) | MI caused by CAS | 8 (0.2) |
| β‐blockers | 270 (4.5) | Coronary revascularization | 15 (0.4) |
| Diuretics | 292 (4.9) | Stroke | 28 (0.6) |
| ARB | 442 (7.5) | Hospitalization because of HF | 32 (0.8) |
| ACEI | 82 (1.3) | MACE | 91 (2.2) |
| Statins | 488 (8.2) | Sustained angina pectoris | 309 (8.1) |
Data are presented as N (%) or mean±SD. MACE was defined as the composite of total death, MI, coronary revascularization, stroke, and hospitalization because of HF. ACEI indicates angiotensin converting enzyme inhibitors; ACH, acetylcholine; ARB, angiotensin receptor blockers; CAS, coronary artery spasm; HF, heart failure; MACE, major adverse cardiac events; MI, myocardial infarction.
Table 2 shows the hazard ratio of various risk factors on MACE or sustained AP by univariate and stepwise multivariable Cox proportional hazards model analysis. We confirmed that the assumption of proportional hazards was met; the presence of nonsignificant coronary lesions such as CAS, MB, and ICS also can lead to MACE or sustained AP at long‐term follow‐up. As a result, MACE was significantly associated with age and ICS. Sustained AP was significantly associated with age, dyslipidemia, ICS, CAS, and MB.
Table 2.
Associations of MACE, Sustained Angina Pectoris, and Risk Factors Using Univariate and Multivariable Cox Proportional Hazards Regression Analysis
| Variables, N (%) | Total | Incidence, % | Univariate | Multivariable | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |||
| MACE up to 5 y | ||||||
| Insignificant stenosis | 848 | 29 (6.0%) | 3.45 (2.21–5.37) | <0.001 | 2.03 (1.28–3.21) | 0.002 |
| 30%–50% | 481 | 14 (5.2%) | 2.55 (1.44–4.51) | 0.001 | 1.84 (1.02–3.32) | 0.043 |
| 50%–70% | 367 | 15 (6.8%) | 3.51 (2.01–6.11) | <0.001 | 2.26 (1.26–4.05) | 0.006 |
| Coronary artery spasm | 3394 | 58 (2.4%) | 1.29 (0.84–1.98) | 0.234 | ··· | ··· |
| Myocardial bridge | 853 | 16 (2.5%) | 1.15 (0.67–1.98) | 0.596 | ··· | ··· |
| Sex, male | 5890 | 91 (2.2%) | 0.99 (0.66–1.50) | 0.999 | ··· | ··· |
| Age, 5 y | 5890 | 55.3±12.4 | 1.50 (1.36–1.66) | <0.001 | 1.44 (1.30–1.60) | <0.001 |
| Hypertension | 2694 | 55 (3.0%) | 1.83 (1.20–2.79) | 0.005 | 1.06 (0.68–1.65) | 0.770 |
| Diabetes mellitus | 928 | 25 (3.7%) | 2.09 (1.32–3.32) | 0.002 | 1.38 (0.86–2.20) | 0.178 |
| Dyslipidemia | 1757 | 36 (3.0%) | 1.58 (1.04–2.41) | 0.032 | 1.25 (0.81–1.92) | 0.294 |
| Current smoking | 1213 | 19 (2.3%) | 0.98 (0.59–1.62) | 0.941 | ··· | ··· |
| Sustained angina pectoris up to 5 y | ||||||
| Insignificant stenosis | 848 | 81 (17.3%) | 2.88 (2.23–3.71) | <0.001 | 2.54 (1.94–3.31) | <0.001 |
| 30%–50% | 481 | 32 (13.3%) | 1.72 (1.19–2.48) | 0.004 | 1.75 (1.20–2.55) | 0.004 |
| 50%–70% | 367 | 49 (22.0%) | 3.81 (2.81–5.18) | <0.001 | 3.63 (2.64–5.01) | <0.001 |
| Coronary artery spasm | 3394 | 208 (9.6%) | 1.53 (1.21–1.95) | <0.001 | 1.42 (1.11–1.80) | 0.004 |
| Myocardial bridge | 853 | 63 (10.0%) | 1.38 (1.04–1.82) | 0.022 | 1.37 (1.04–1.81) | 0.024 |
| Sex, male | 5890 | 153 (8.7%) | 1.14 (0.91–1.43) | 0.226 | ··· | ··· |
| Age, 5 y | 5890 | 55.3±12.4 | 1.10 (1.05–1.16) | <0.001 | 1.05 (1.01–1.11) | 0.026 |
| Hypertension | 2694 | 157 (8.8%) | 1.25 (1.00–1.56) | 0.050 | 0.98 (0.77–1.24) | 0.902 |
| Diabetes mellitus | 928 | 58 (9.8%) | 1.28 (0.96–1.70) | 0.088 | 1.04 (0.77–1.39) | 0.792 |
| Dyslipidemia | 1757 | 117 (10.1%) | 1.51 (1.20–1.90) | <0.001 | 1.34 (1.06–1.70) | 0.013 |
| Current smoking | 1213 | 73 (8.6%) | 1.15 (0.88–1.50) | 0.281 | ··· | ··· |
MACE was defined as the composite of total death, myocardial infarction, coronary revascularization, stroke, and hospitalization because of heart failure. HR indicates hazard ratio; MACE, major adverse cardiac events.
Figure 2A and 2B shows the 5‐year clinical outcome for MACE or sustained AP according to the combination of CAS, MB, and ICS. Patients without CAS, MB, and ICS had a MACE of 1.1%, but the patients with ICS had a MACE of 3.5% to 7.8% at 5‐year follow‐up (Figure 2A). Additionally, the patients without CAS, MB, and ICS had a sustained AP of 4.7%, but the patients with ICS had a sustained AP of 10.6% to 36.1% at 5‐year follow‐up (Figure 2B).
Figure 2.
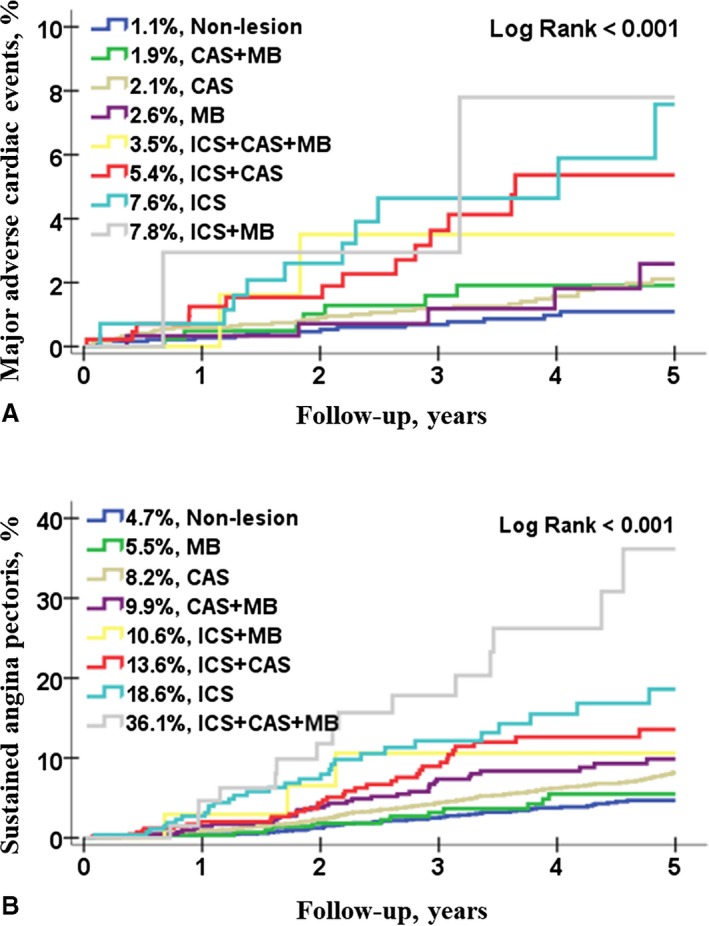
Cumulative 5‐year clinical outcomes in patients without significant coronary artery disease. A, The 5‐year cumulative incidence of major adverse cardiac events (MACE); B shows the 5‐year cumulative incidence of sustained angina pectoris according to the combination of coronary artery spasm (CAS), myocardial bridge (MB), and insignificant coronary stenosis (ICS). MACE was defined as the composite of total death, myocardial infarction, coronary revascularization, stroke, and hospitalization because of heart failure.
Discussion
The main findings of this study are summarized as follows: (1) among the patients with chest pain without significant CAD, 68.3% had CAS, MB, and/or ICS; (2) of these patients, within the 5‐year follow‐up, MACE was occurring in 2.2%, and the main cause of MACE was hospitalizations because of HF (0.8%) and stroke (0.6%); sustained AP was occurring in 8.1% despite antiangina treatments; and (3) on multivariable Cox proportional hazards regression analysis, the main causes of MACE were related to aging and ICS; additionally, aging and ICS, along with dyslipidemia, MB, and CAS, were related to the chief causes of sustained AP. In particular, ICS was seen as the strongest risk factor for MACE and sustained AP.
Obstructive coronary stenosis is a well‐known cause of ischemic heart disease such as AP, MI, ACS, and even sudden cardiac death. A majority of these patients present with chest pain.1, 2, 9, 10 The risk factors and prognoses of these patients are being studied extensively. However, among patients who complain of chest pain without gastrointestinal disease, a large number of patients also appear to have normal coronary arteries in the CAG test.1, 2, 3, 5, 6, 9, 10 Data on the causes or prognoses of these patients are, however, currently limited. Patients in this category are mostly known to have a poor prognosis.8, 10, 11 The range of patients with normal coronary arteries in the ACS group was reported to be as high as 20% to 28%.10, 12 In the CASPAR (Coronary Artery Spasm in Patients With Acute Coronary Syndrome) study, it was reported that ACS patients without culprit lesion and proof of CAS have an excellent prognosis for survival at 3 years compared with patients with obstructive ACS (1% versus 19%; P<0.01).10 In contrast, in a Korean acute MI registry study, patients with near‐normal coronary angiograms had similar survival rates compared with patients with 1‐ or 2‐vessel disease presenting with an acute MI (2.6% versus 2.2%; P=0.952).10 Therefore, it is necessary to clearly evaluate the long‐term clinical outcome and its association in patients with chest pain without significant CAD.
In the present study, among the patients with chest pain without significant CAD, 57% had CAS, MB (14%), and/or ICS (14%). Although many studies have shown normal coronary arteries in a similar population, a high rate of CAS has been reported.10, 12, 13 Regarding patients with ACS, the CASPAR study in Germany and the United Kingdom reported that 28% (138/488) of patients were without any culprit lesions and 48% (37/76) of these patients were diagnosed with epicardial CAS on performing the ACH test; Satoh et al12 reported that, in their study, 20% (130/645) patients had no coronary stenosis and 54% (70/130) of these patients were diagnosed with CAS on performing the ACH test.10, 12 Similar to our study population, Ishii et al13 reported that 79% (1402/1760) of the patients with typical or atypical angina‐like chest pain were determined to have no significant organic stenosis, and 45% (640/1402) of these patients were diagnosed with CAS on performing the ACH test. Our prior studies have also reported that MB and ICS were significantly related to CAS.3, 6 Therefore, the results of previous studies and our study show that CAS, MB, and ICS are the major causes of chest pain in patients who present with chest pain without significant CAD.3, 5, 6, 9, 10, 12, 13 However, the incidence of CAS varies according to ethnicity, social characteristics, provocation methods, and definition of CAS.14 Traditionally, it is known that Far Eastern countries (Japan, 40.9%–79%) have reported a higher incidence of CAS than Western countries (France, 15.5%; Germany, 49%) by ACH test. The source data of our study was a Korean population at a single center, so the results of this study cannot be generalized to all ethnicities.
Our study excluded patients with a history of cardiovascular disease, stroke, and HF, so the risk of cardiovascular disease seemed to be relatively low. Nevertheless, of these patients, within the 5‐year follow‐up, patients with sustained AP (8.1%) and MACE (2.2%) were hospitalized. On performing multivariable Cox proportional hazards regression analysis, the incidence of MACE was seen to be correlated with aging and ICS. Similarly, sustained AP was found to be correlated with aging, dyslipidemia, ICS, MB, and CAS within 5 years of follow‐up. Similar to our study results, the CASPAR study and the study by Bory et al15 reported that patients without culprit lesions have an excellent prognosis for survival in spite of persistent or recurrent episodes of angina.9, 10, 15 However, some studies have reported tragic results such as MI, sudden death, or poor long‐term outcomes.8, 10, 11 Kim et al8 reported that MI caused by CAS are rare (1.01%; 34/3360), but the occurrence of MACE is higher in patients with MI caused by CAS than in CAS patients without MI (1.5% versus 13.3%; hazard ratio, 10.9; 95% CI, 3.8–31.2; P<0.001). Nishizawa et al16 reported that ICS at the CAS site is an independent association for long‐term major cardiac events (hazard ratio, 4.5, 95% CI, 1.7–11.9; P=0.002) in patients with ergonovine‐induced CAS. Therefore, evaluation of long‐term clinical outcomes with CAG results and ACH tests is required. In the present study, which was a long‐term clinical evaluation of MACE after a combination of CAS, MB, and ICS, all combinations of ICS are seen as the strongest association for both MACE and sustained AP. Additionally, during the 5‐year follow‐up period stroke (0.6%) and HF (0.8%) were the most frequent events. Among the patients with chest pain without significant CAD, it is necessary to carefully evaluate the risk of stroke and HF.
This study has several limitations. First, the ACH test can identify epicardial CAS, but microvascular CAS confirmation should be symptomatic. In this study, only epicardial CAS was defined as CAS. Second, previous studies have defined CAS from 70% narrowing of the coronary artery to subtotal or total occlusion.5, 6, 7, 17 In the Japanese Circulation Society's guidelines for diagnosis and treatment of patients with vasospastic angina, a significant CAS is defined as transient, total, or subtotal occlusion (>90% stenosis) of the artery during the drug‐induced CAS provocation test.17 Though 70% narrowing may be less stringent than total occlusion, the more severe the spasm, the higher would be the chance of hemodynamic instability and advanced atrioventricular block during the ACH provocation test. Because most of the ACH provocation tests were performed at the outpatient base by the 4F radial approach, patient safety was regarded as the first priority. Third, routine myocardial stress tests such as single‐photon emission computed tomography, exercise treadmill, and dobutamine stress echocardiography were not performed because of the assumption of a limited possibility of significant fixed coronary artery stenosis in patients suffering from mainly resting ischemic chest pain suspicious for vasospastic angina, not effort‐induced angina. Finally, we could not gather any detailed follow‐up data on antiangina medication during the follow‐up. However, all patients received antiangina medication until they were free of angina symptoms and in clinical remission, although the medication type and duration were based on the discretion of the individual physicians. Further well‐designed and longer‐term follow‐up studies are needed to obtain more accurate answers to all these questions.
Disclosures
None.
(J Am Heart Assoc. 2019;8:e010541 DOI: 10.1161/JAHA.118.010541.)
References
- 1. Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, Ferguson TB Jr, Fihn SD, Fraker TD Jr, Gardin JM, O'Rourke RA, Pasternak RC, Williams SV, Gibbons RJ, Alpert JS, Antman EM, Hiratzka LF, Fuster V, Faxon DP, Gregoratos G, Jacobs AK, Smith SC Jr; American College of Cardiology, American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Chronic Stable A) . ACC/AHA 2002 guideline update for the management of patients with chronic stable angina—summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients With Chronic Stable Angina). Circulation. 2003;107:149–158. [DOI] [PubMed] [Google Scholar]
- 2. Yasue H, Nakagawa H, Itoh T, Harada E, Mizuno Y. Coronary artery spasm—clinical features, diagnosis, pathogenesis, and treatment. J Cardiol. 2008;51:2–17. [DOI] [PubMed] [Google Scholar]
- 3. Nam P, Choi BG, Choi SY, Byun JK, Mashaly A, Park Y, Jang WY, Kim W, Choi JY, Park EJ, Na JO, Choi CU, Lim HE, Kim EJ, Park CG, Seo HS, Oh DJ, Rha SW. The impact of myocardial bridge on coronary artery spasm and long‐term clinical outcomes in patients without significant atherosclerotic stenosis. Atherosclerosis. 2018;270:8–12. [DOI] [PubMed] [Google Scholar]
- 4. Kim JW, Park CG, Suh SY, Choi CU, Kim EJ, Rha SW, Seo HS, Oh DJ. Comparison of frequency of coronary spasm in Korean patients with versus without myocardial bridging. Am J Cardiol. 2007;100:1083–1086. [DOI] [PubMed] [Google Scholar]
- 5. Ong P, Athanasiadis A, Borgulya G, Vokshi I, Bastiaenen R, Kubik S, Hill S, Schaufele T, Mahrholdt H, Kaski JC, Sechtem U. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive white patients with unobstructed coronary arteries. Circulation. 2014;129:1723–1730. [DOI] [PubMed] [Google Scholar]
- 6. Choi BG, Park SH, Rha SW, Ahn J, Choi SY, Byun JK, Li H, Mashaly A, Shim MS, Kang JH, Kim W, Choi JY, Park EJ, Lee S, Na JO, Choi CU, Lim HE, Kim EJ, Park CG, Seo HS, Oh DJ. Three‐year follow‐up of patients with acetylcholine‐induced coronary artery spasm combined with insignificant coronary stenosis. Int J Cardiol. 2017;238:66–71. [DOI] [PubMed] [Google Scholar]
- 7. Choi BG, Jeon SY, Rha SW, Park SH, Shim MS, Choi SY, Byun JK, Li H, Choi JY, Park EJ, Park SH, Lee JJ, Lee S, Na JO, Choi CU, Lim HE, Kim JW, Kim EJ, Park CG, Seo HS, Oh DJ. Impact of renin‐angiotensin system inhibitors on long‐term clinical outcomes of patients with coronary artery spasm. J Am Heart Assoc. 2016;5:e003217 DOI: 10.1161/JAHA.116.003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim JB, Choi BG, Rha SW, Seo HS, Choi SY, Byun JK, Na JO, Choi CU, Kim EJ, Park CG, Oh DJ. Clinical characteristics and outcomes of patients with coronary artery spasm who initially presented with acute myocardial infarction. Coron Artery Dis. 2018;29:60–67. [DOI] [PubMed] [Google Scholar]
- 9. Ong P, Athanasiadis A, Hill S, Vogelsberg H, Voehringer M, Sechtem U. Coronary artery spasm as a frequent cause of acute coronary syndrome: the CASPAR (Coronary Artery Spasm in Patients With Acute Coronary Syndrome) study. J Am Coll Cardiol. 2008;52:523–527. [DOI] [PubMed] [Google Scholar]
- 10. Kang WY, Jeong MH, Ahn YK, Kim JH, Chae SC, Kim YJ, Hur SH, Seong IW, Hong TJ, Choi DH, Cho MC, Kim CJ, Seung KB, Chung WS, Jang YS, Rha SW, Bae JH, Cho JG, Park SJ; Korea Acute Myocardial Infarction Registry Investigators . Are patients with angiographically near‐normal coronary arteries who present as acute myocardial infarction actually safe? Int J Cardiol. 2011;146:207–212. [DOI] [PubMed] [Google Scholar]
- 11. Laporte F, Moulin F, Brembilla‐Perrot B. Sudden death caused by atypical variant angina. Arch Cardiovasc Dis. 2011;104:480–481. [DOI] [PubMed] [Google Scholar]
- 12. Satoh S, Omura S, Inoue H, Mori T, Takenaka K, Numaguchi K, Mori E, Aso A, Nakamura T, Hiyamuta K. Clinical impact of coronary artery spasm in patients with no significant coronary stenosis who are experiencing acute coronary syndrome. J Cardiol. 2013;61:404–409. [DOI] [PubMed] [Google Scholar]
- 13. Ishii M, Kaikita K, Sato K, Tanaka T, Sugamura K, Sakamoto K, Izumiya Y, Yamamoto E, Tsujita K, Yamamuro M, Kojima S, Soejima H, Hokimoto S, Matsui K, Ogawa H. Acetylcholine‐provoked coronary spasm at site of significant organic stenosis predicts poor prognosis in patients with coronary vasospastic angina. J Am Coll Cardiol. 2015;66:1105–1115. [DOI] [PubMed] [Google Scholar]
- 14. Choi BG, Park SH, Rha SW, Park JY, Choi SY, Park Y, Xu S, Ngow HA, Ali J, Li H, Kim JB, Lee S, Na JO, Choi CU, Lim HE, Kim JW, Kim EJ, Park CG, Seo HS, Oh DJ. Five‐year clinical outcomes in patients with significant coronary artery spasm: a propensity score‐matched analysis. Int J Cardiol. 2015;184:533–539. [DOI] [PubMed] [Google Scholar]
- 15. Bory M, Pierron F, Panagides D, Bonnet J, Yvorra S, Desfossez L. Coronary artery spasm in patients with normal or near normal coronary arteries long‐term follow‐up of 277 patients. Eur Heart J. 1996;17:1015–1021. [DOI] [PubMed] [Google Scholar]
- 16. Nishizawa S, Shiraishi J, Torii S, Miyagawa K, Arihara M, Hadase M, Hyogo M, Yagi T, Shima T, Kohno Y, Matsubara H. Intermediate fixed coronary artery stenosis at the site of ergonovine‐provoked spasm as a predictor of long‐term major adverse cardiac events of patients with coronary spastic angina. Circ J. 2009;73:699–704. [DOI] [PubMed] [Google Scholar]
- 17. Group JCSJW . Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ J. 2014;78:2779–2801. [DOI] [PubMed] [Google Scholar]


