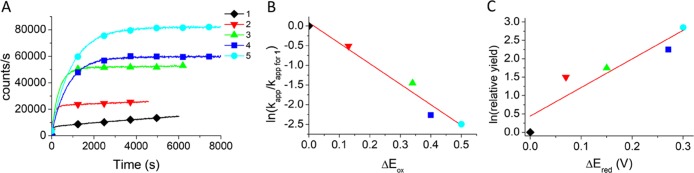
Figure 3.
(A) Intensity–time trajectories following the reaction of compounds 1–5 with 1.09 M methanol in acetonitrile supplemented with 0.333 mM of p-TsOH at 21 °C. Dye concentrations were prepared such that their respective absorbance was 0.1 at the excitation wavelength used (i.e., 16, 20, 4.5, 16, and 3 μM for dyes 1–5, respectively) (B) LFER correlation (slope = −5.2 and intercept = 0.1) between the ratio of apparent rate constants (kapp) and the difference in oxidation potentials (to compound 1) in acetonitrile for BODIPY dyes 1–5 vs ferrocene previously obtained by us.18 Where reversible oxidation potentials were not available (i.e., compounds 3–5), anodic peak potentials were used. Compound 1kapp and oxidation potentials were used as a reference. (C) LFER correlation (slope = 8 and intercept = 0.4) between the calculated relative yield (vide infra) and the difference in reversible reduction potentials in acetonitrile for dyes 1–5 (relative to compound 1) measured vs ferrocene previously obtained by us.18 Compound 1 yield and reduction potentials were used as a reference.