Abstract
INTRODUCTION:
We evaluated whether incident mild cognitive impairment (MCI) subtypes could be empirically derived in the Mayo Clinic Study of Aging (MCSA).
METHODS:
We performed cluster analysis on neuropsychological data from 506 participants with incident MCI.
RESULTS:
The 3 cluster solution resulted in: (1) amnestic; (2) dysexecutive; (3) dysnomic subtypes. The 4 cluster solution produced these same three groups and a fourth group with subtle cognitive impairment (SCI). The SCI cluster was a subset of the amnestic cluster and distinct from well-matched CU participants based on memory and global z-score AUROC analyses and probability of progression to MCI/dementia.
DISCUSSION:
We empirically identified three neuropsychological subtypes of MCI that share some features with MCI subtypes identified in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). The fourth subtype with SCI in the MCSA differed from fourth the cluster-derived normal group in ADNI and could represent a group to target with early interventions.
Keywords: Mild cognitive impairment, MCI, Neuropsychology, Cluster analysis, Subtle cognitive impairment
1. Introduction
The aging and dementia field is increasingly focused on identifying and characterizing the earliest and subtlest cognitive changes that occur as individuals transition from cognitively unimpaired (CU) to mild cognitive impairment (MCI), especially since the introduction of “subtle cognitive/behavioral decline” (in addition to amyloidosis and neuronal injury) as one of the features of preclinical Alzheimer’s disease [1]. A new National Institute on Aging and Alzheimer’s Association research framework was introduced in 2018 [2]. This framework uses a numeric clinical staging scheme with six stages to describe individuals in the Alzheimer’s continuum with Stage 2 characterized by “transitional cognitive decline” defined by a decline in previous level of function despite normal performance within the expected range on objective cognitive tests. Conceptually, this is similar to “Stage 3 preclinical AD” from the 2011 NIA-AA guidelines.
In an attempt to more fully operationalize the spectrum of MCI in a non-biased manner, several studies have used empirical, and specifically cluster-analytic techniques, on neuropsychological test data rather than theoretical pre-specified cut-points in one or more cognitive domains. Delano-Wood (2009) [3] was one of the first to provide evidence for three distinct groups of MCI in their clinic-based sample: memory/language, executive/processing speed, and pure memory. These subtypes also showed a dissociation of white matter lesion type in the two most impaired groups, with the memory/language group showing higher periventricular lesions and the executive/processing speed group showing higher deep white matter lesions. Libon and colleagues [4] performed a cluster analysis on patients self-referred to an outpatient memory clinic and diagnosed with single- and multi-domain MCI based on subjective complaints of cognitive decline, ≤ 24 on the MMSE [5], no impairment in activities of daily living and a neuropsychological test performance of ≤ 1.5 SD on any of six neuropsychological tests [6]. Their cluster analysis revealed a group of patients with amnestic MCI, a second with dysexecutive MCI, and a third with mixed/multi-domain MCI. This study supported previous work suggesting the existence of single domain and multi-domain MCI subtypes [7]. Another study using hierarchical cluster analysis on a large sample of patients from twenty memory clinics who presented with subjective or objective memory impairment also found evidence for single- and multi-domain amnestic MCI subtypes as well as another group with subjective cognitive impairments and very mild to no objective cognitive deficits [8].
Additional studies using cluster analytic techniques have also identified individuals with normal cognition who were diagnosed with MCI. For example, Clark et al [9] compared conventional (i.e., ≤ 1.5 SD below normal on one test with a domain)[6] vs. comprehensive criteria (< 1 SD below normal in two tests within a domain) [10] in a community-based sample of patients with prevalent MCI. Both criteria revealed an amnestic subtype and mixed subtype that captured individuals with advanced stages of MCI given their impaired scores on measures of memory, executive function, language and visuospatial function. The comprehensive criteria also yielded dysexecutive and visuospatial subtypes while the conventional criteria produced a cluster-derived normal group. Bondi et al [11] applied conventional versus comprehensive criteria for defining MCI in the ADNI cohort. They again found that both criteria produced a mildly impaired amnestic subtype and a more severely impaired dysexecutive/mixed subtype. The comprehensive criteria also uniquely identified a language subtype whereas the conventional criteria produced a third subtype of individuals (which comprised nearly a third of the sample) performing within normal limits. Edmonds et al. also performed a cluster-analysis on 825 ADNI participants diagnosed with MCI at their initial screening based on ADNI diagnostic criteria [12] and identified three subtypes of MCI in the ADNI sample: (1) dysnomic, (2) dysexecutive, (3) amnestic as well as fourth cluster-derived normal group [13]. (See Appendix 1.)
Appendix 1.
Cluster Analysis Studies on MCI
| Reference | Sample/Source | NP Tests | MCI definition | Results |
|---|---|---|---|---|
| Delano-Wood, Bondi, Sacco et al. JESTS, 2009.15:906–914. | 70 older adults/Geriatric Neurology Clinic | CERAD battery; Trail Making Test, Parts A & B, Stroop Color-Word Test | ≥ 1.2 SD below the mean on one more tests after applying norms adjusted for age, education, and gender | 3 clusters: Executive/Processing Speed, Memory/Language, Pure Memory |
| Libon, Xie, Eppig, et al. JINS, 2010.16:84–93. | 108 outpatients describing a decline in cognitive functioning/Geriatric Neurology Clinic | WMS Mental Control subtest (Boston Revision), Letter fluency, Boston Naming Test, Animal Fluency, Philadelphia Verbal Learning Test Delay Free Recall/Recognition | ≥ 1.5 SD below the mean on any of the 6 neuropsychological tests | 3 clusters: Amnesic MCI, Dysexecutive MCI, Mixed MCI |
| Damian, Hausner, Jekel, et al. Dement Geriatr Cogn Disord, 2013.36:1–19. | 881 subjects with objective or subjective cognitive impairment. DESCRIPA study; 20 European memory clinics | AVLT, CERAD Word List, Animal fluency, Trail Making Test, Parts A & B, Rey-Osterrieth Complex Figure, CERAD Figure Copy. Note: NP tests administered varied among centers | z-scores> ≥−1.5 or≥ −1.0 were used to define impaired test performance. Diagnoses were made on the basis of a clinical consensus. | 4 clusters: Subjective cognitive impairment, Inattentive/dysexecutive aMCI, Dysexecutive aMCI, aMCI, single domain |
| Clark, Delano-Wood, Libon, et al. JINS, 2013. 19:635–645. | 197 non-demented older adults/Longitudinal study of aging at the UCSD/VA San Diego/UCSD ADRC | CVLT, WMS-R Logical Memory, Trail Making Test Parts A & B, D-KEFS CWIT-Inhibition/Switching, WCST (48-Card Version), Boston Namin Test, Letter Fluency, Animal Fluency, WISC-R Block Design, WMS-R Visual Reproduction I/II, DRS Construction, Clock Drawing, WAIS-R Digit Span Forward, D-KEFS Visual Scanning, DRS Attention | 134 diagnosed with MCI based on conventional criteria: ≥ 1.5 SD below the mean on at least one measure in the NP battery 80 were diagnosed with MCI based on comprehensive criteria: ≥ 1 SD below the mean on at least 2 measures within a cognitive domain | Conventional MCI criteria (3 clusters): Amnestic/language, Mixed, Cluster derived Normal Comprehensive MCI criteria (4 clusters): Amnestic, Mixed, Dysexecutive, Visuospatial |
| Bondi, Edmonds, Jak, et al. J Alz Dis, 2014. 42:275–289. | 846 with MCI based on ADM criteria 401 MCI defined with Jak/Bondi criteria/ADNI participants | AVLT, Animal Fluency, Boston Naming Test (30-items), Trail Making Test Parts A & B | Reclassified using Jak/Bondi actuarial neuropsychological test methods: 1) > 1 SD below the age-corrected mean on both measures within at least one cognitive domain; 2) > 1 SD below the mean on one test in each of the three cognitive domains; 3) FAQ = 9 | ADNI criteria (3 clusters): Amnestic, Dysexecutive/Mixed MCI, Cluster-Derived Normal Jak/Bondi Criteria (3 clusters): Amnestic, Language, Dysexecutive/Mixed |
| Edmonds, Delano-Wood, Clark, et al. Alz & Dem, 2015. 11:415–424 | 825 MCI/ADNI participants | AVLT, Animal Fluency, Boston Naming Test (30-items), Trail Making Test Parts A & B | 1) Subjective memory complaint reported by participant or study partner; 2) MMSE 24–30 (inclusive); 3) global CDR = 0.5; 4) Scoring below education-adjusted cutoffs on WMS-R Logical Memory II; 5) General cognition/functional performance not qualifying for dx of AD | 4 clusters: Amnestic, Dysnomic, Dysexecutive, Cluster-Derived Normal |
The evidence thus far suggests there are subtypes of prevalent MCI that can be empirically identified. The most frequently identified subtypes are amnestic and dysexecutive MCI [3, 4, 8, 9, 11, 13] with two studies also identifying a language subtype on the ADNI dataset [11, 13] and one identifying a visuospatial subtype in a community-based sample [9]. The clusters are contingent upon the neuropsychological measures included in the analyses as well as criteria used to operationalize MCI. Some studies show that the over-sensitivity of conventional diagnostic criteria may result in misclassification of individuals as having MCI when in fact these individuals are CU [9, 11, 13]. A limitation of the studies done to date is that they are based on prevalent MCI and impairment in some cognitive domains may have progressed further for some individuals with MCI compared to others. This study expands on the research to determine the reproducibility of empirically derived MCI subtypes in a population-based sample and to characterize the cognitive changes that occur in incident MCI. The objective of this study was to use cluster analysis to identify neuropsychological subtypes of incident MCI in the Mayo Clinic Study of Aging [14].
2. Methods
2.1. Study sample
2.1.1. Cluster Analysis Participants
Participants were from the Mayo Clinic Study of Aging (MCSA) which is a longitudinal population-based study of cognitive aging in Olmsted County, Minnesota [14]. All participants were ≥ 50 years old at their baseline assessment and classified as cognitively unimpaired (CU). Given the emphasis on evaluating cognitive changes that occur as participants transition from CU to MCI, we first identified a cohort of participants with incident MCI. We required that all MCI participants have at least one prior visit at which they were classified as CU. We also required that all MCI participants have at least one subsequent visit after the visit at which they were diagnosed with incident MCI so that we could examine reversion rates. The Mayo Clinic and Olmsted Medical Center Institutional Review Boards approved these studies which also followed Health Insurance Portability and Accountability Act (HIPAA) guidelines. Every participant provided written informed consent.
2.2. Materials and Procedure
2.2.1. Evaluation
Participants completed comprehensive evaluations at approximately 15 month intervals which included a physician examination, an interview by a study coordinator, and neuropsychological testing [14]. The physician examination included a medical history review, complete neurologic examination, and administration of the Short Test of Mental Status [15]. The study coordinator interview included demographic information, medical history, and questions about memory to the participant using the Blessed Memory Test [16] and the informant using the Clinical Dementia Rating (CDR) Scale [17] and the Functional Activities Questionnaire (FAQ) [18].
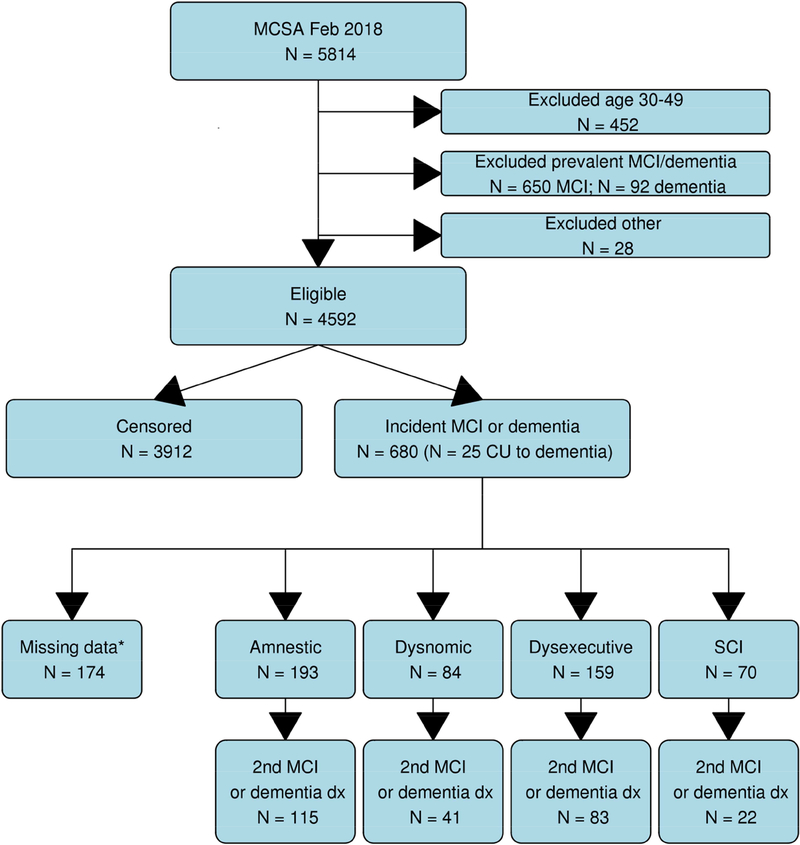
Neuropsychological testing included nine measures assessing four cognitive domains: (1) Memory (AVLT Delayed Recall [19], WMS-R Logical Memory II & Visual Reproduction II) [20], (2) Language (Boston Naming Test [21], Category Fluency [22]), (3) Attention/Executive (Trailmaking Test B [22, 23], WAIS-R Digit Symbol [24]), (4) Visuospatial (WAIS-R Picture Completion & Block Design [24]). For each participant, cognitive performance in each domain was compared with age-adjusted scores of individuals previously obtained using Mayo’s Older American Normative Studies [25–27]. This approach relies on prior normative work and extensive experience with the measurement of cognitive abilities in an independent sample of participants from the same population. Given that we were clustering participants on neuropsychological test data, we had the strict requirement that all participants have data from ≥ 8 of the 9 cognitive tests administered at each study visit. This resulted in excluding 174 participants. (See Figure 1).
Figure 1. Flow chart outlining steps used to establish the study sample.
* Ineligible for clustering due to missing data
The criteria used to diagnose MCI were those described in the paper by Petersen [7] and follow the outline above, with history from the participant and interview of a study partner to determine if there has been 1) a change in cognition, 2) objective scores in the −1.0 SD below the mean range that the clinicians believe are below what would be expected for that individual in one or more cognitive domains based on the normative data we use, 3) functionally intact, and 4) does not meet DSM-IV criteria for dementia. In addition, these criteria are consistent with the recent evidence-based review of the literature [28]. A final decision to diagnose CU or MCI was based on a consensus agreement among study coordinator, examining physician, and neuropsychologist, after taking into account education, prior occupation, visual or hearing deficits, and reviewing all other participant clinical information [7, 14]. A diagnosis of dementia was based on published criteria [29]. All raters are blinded to the previous diagnosis of the participant.
2.2.2. Genetic characterization
All participants underwent a blood draw at their baseline visit. DNA extraction and apolipoprotein E (APOE) genotyping was performed for each participant using standard methods [30]. The APOE ε4 carrier group included participants with one or two copies of the ε4 allele (i.e., ε2ε4, ε3ε4, ε4ε4).
2.3. Statistical analyses
2.3.1. Cluster analysis
Neuropsychological test z-scores were computed and averaged by domain and referenced to 3686 MCSA 2004–2012 CU from the 50–89 cohort and weighted to the 2013 Olmsted County population by age and sex. We performed agglomerative hierarchical clustering with Euclidean distance and Ward’s linkage on the MCI participants’ neuropsychological domain z-scores [31]. Based on our desire to capture a reasonably sized, fairly mild MCI group we determined that four clusters were better than three. We conducted a discriminant function analysis to quantitatively examine the ability of the cognitive domain scores to discriminate the cluster subgroups. The stability of the cluster solution was also evaluated using the leave-one-out cross-validation procedure which minimizes the potential bias of using the same participants to develop the cluster solution as used to compute the discriminant function[32]. We then calculated analysis of variance (ANOVA) or chi-square goodness of fit test to assess group differences in baseline demographic features.
2.3.2. AUROC
We calculated the area under the receiver operating characteristic curve (AUROC) as a nonparametric measure of effect size [33] and calculated 95% confidence intervals for each AUROC estimate.[34]
2.3.3. Cox Proportional Hazards
We also considered the probability of diagnosing participants as MCI at their future visit.. To do this, we compared the proportion of CU participants with incident MCI or dementia, and the proportion of the MCI groups having a confirmatory diagnosis of MCI or dementia (i.e., a diagnosis of MCI or dementia at the next visit.) P-values to assess pairwise group differences were attained by fitting a Cox Proportional Hazards model on time to recurrence of MCI or dementia with age as the time-scale and adjusting for sex. To correctly account for multiple events per person, robust standard errors were estimated using the Huber sandwich estimator. We entered cluster membership as a time-dependent covariate with group membership assigned at first occurrence of MCI or dementia. All analyses were completed in R version 3.4.2. (https://www.r-project.org)
3. Results
3.1. Demographics
This study included 506 participants who received a diagnosis of incident MCI. Figure 1 provides a flow chart of the steps used to derive the study sample. Table 1 provides demographics and clinical characteristics for the four cluster solution and the CU group.
Table 1.
Demographics of incident MCI clusters referenced to 50+ CU weighted to Olmsted County
| Amnestic (N=193) | Dysnomic (N=84) | Dysexecutive (N=159) | SCI (N=70) | p value* | CU† (N=3912) |
|
|---|---|---|---|---|---|---|
| Age, yrs | ||||||
| Median (Q1,Q3) | 82 (76, 86) | 86 (80, 89) | 84 (81, 88) | 81 (76, 84) | < 0.001‡ | 72 (63, 78) |
| Education, yrs | ||||||
| Median (Q1,Q3) | 13 (12, 16) | 12 (12, 14) | 13 (12, 15) | 15 (12, 18) | < 0.001‡ | 14 (12, 16) |
| Sex | ||||||
| F=FEMALE | 90 (47%) | 48 (57%) | 71 (45%) | 30 (43%) | 0.231§ | 1988 (51%) |
| CDR Sum of Boxes | ||||||
| Median (Q1,Q3) | 0.5 (0, 1) | 0.5 (0, 1.5) | 1 (0, 1.5) | 0.5 (0, 1) | 0.009‡,¶,# | 0 (0, 4) |
| APOE ε4 carrier | ||||||
| 1=Yes | 73 (38%) | 29 (35%) | 59 (38%) | 23 (33%) | 0.857§ | 900 (25%) |
| STMS Total | ||||||
| Median (Range) | 31 (25, 38) | 29 (19, 36) | 30 (22, 36) | 33 (26, 37) | < 0.001‡ | 36 (34, 37) |
| MCSA cycle | ||||||
| Median (Q1,Q3) | 3 (2, 4) | 3 (2, 5) | 3 (2, 4) | 4 (3, 5) | 0.023‡ | 1 (1, 1) |
| Global z | ||||||
| Median (Q1,Q3) | −1.4 (−1.7, −1.1) | −2.7 (−3.5, −2.2) | −2.4 (−2.8, −1.8) | −0.4 (−0.8, −0.1) | < 0.001‡ | 0.1 (−0.6, 0.7) |
| Memory z | ||||||
| Median (Q1,Q3) | −1.8 (−2.2, −1.2) | −2.1 (−2.6, −1.7) | −1.3 (−1.9, −0.6) | −0.6 (−1.7, −0.1) | < 0.001‡ | −0.0 (−0.7, 0.7) |
| Language z | ||||||
| Median (Q1,Q3) | −1.1 (−1.5, −0.6) | −3.1 (−3.8, −2.5) | −1.4 (−1.9, −0.9) | −0.1 (−0.6, 0.3) | < 0.001‡ | 0.0 (−0.6, 0.7) |
| Attention z | ||||||
| Median (Q1,Q3) | −0.9 (−1.6, −0.4) | −2.0 (−3.1, −1.4) | −3.4 (−3.8, −2.8) | −0.4 (−0.8, 0.0) | < 0.001‡ | 0.1 (−0.6, 0.7) |
| Visuospatial z | ||||||
| Median (Q1,Q3) | −0.7 (−1.2, −0.3) | −1.7 (−2.4, −1.3) | −1.4 (−2.0, −0.7) | 0.2 (−0.7, 0.7) | < 0.001‡ | 0.1 (−0.6, 0.7) |
| FAQ Total (0–30) | ||||||
| Median (Q1,Q3) | 0 (0, 2) | 0 (0, 4) | 1 (0, 5) | 0 (0, 1) | < 0.001‡ | 0 (0, 0) |
p value testing differences among the 4 clusters;
censored CU participants; IQR = interquartile range
Linear Model ANOVA;
Pearson’s Chi-squared test;
Wilcoxan Rank Sum, Dysnomic < SCI;
Wilcoxan Rank Sum, Dysexecutive < SCI; SCI = Subtle cognitive impairment; CDR = Clinical Dementia Rating scale; STMS = Short Test of Mental Status; FAQ = Functional Activities Questionnaire
3.2. Cluster Analysis
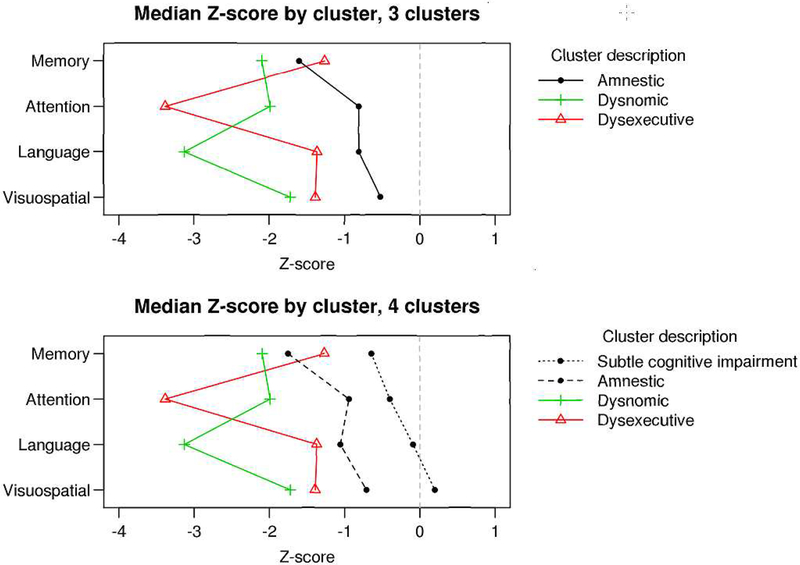
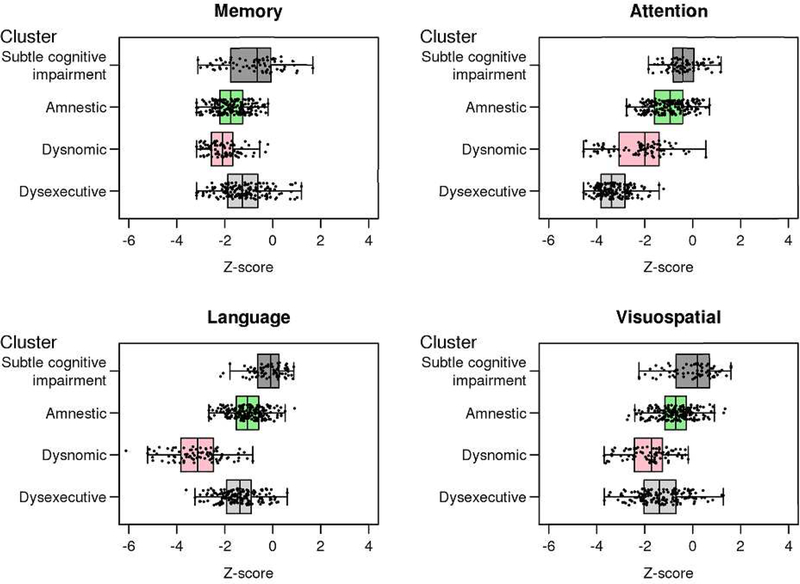
Given that we wanted to examine incident MCI, the cluster assignment occurred at the first diagnosis of MCI. The 3 cluster solution produced the following groups: (1) amnestic (n = 263); (2) dysexecutive (n = 159); (3) dysnomic (n = 84). The 4 cluster solution produced these same three clusters with comparable performance in the four cognitive domains and a fourth cluster labeled as subtle cognitive impairment (SCI; n = 70) that was a subset of the amnestic cluster and distinct from the other groups with respect to level of cognitive performance and degree of functional impairment. This resulted in 193 participants in the amnestic cluster in the 4 cluster solution. Figure 2 shows the median z-score by domain for the 3 and 4 cluster solutions. Figure 3 shows the boxplots of neuropsychological domains z-scores for the four cluster solution. The 5 cluster solution was comparable to the 4-cluster solution with the exception of an additional cluster that essentially replicated the dysexecutive subtype.
Figure 2. Plots of median Z-scores for by domain for 3 cluster and 4 cluster solutions.
The x-axis represents the median Z-score for each cognitive domain by cluster. The cognitive domains are represented on the y-axis.
Figure 3. Boxplots of neuropsychological domain z-scores for the 4 cluster solution.
The x-axis represents the z-scores and the y-axis represents each cluster derived from the 4-cluster solution.
The amnestic, dysexecutive, and dysnomic clusters had a reversion rate to CU of ~ 30% at the next visit whereas SCI cluster had a reversion rate of ~70% which is not unexpected given that the degree of cognitive change is much milder than the other clusters and the blindedness of the evaluators to previous performance.
3.3. Discriminant Function Analysis
We performed linear discriminant analysis which showed that the four cluster solution model accurately classified 87% of the participants. A leave-one-out cross validation estimated accuracy at 86%, indicating a bias of overfitting.
3.4. Matching Procedure
In order to examine whether participants in the SCI cluster differed from CU individuals, we identified 5 CU controls for each SCI case, matching on age (+/− 5 years), sex (exact), and number of exposures to neuropsychological tests (exact for 1 to 5+) from the group of Censored individuals in Figure 1. The five year caliper was generous – most of our participants were exactly matched on visit. In order to get 5 CU matches, we allowed CU at the second visit to match the SCI cluster at the third visit. This matching procedure allowed us to correct for any demographic differences that might explain our results. The demographic information of the SCI cluster matched to CU participants is provided in Appendix 2.
Appendix 2.
Demographics of the incident subtle cognitive impairment cluster matched 5:1 to 350 cognitively unimpaired participants referenced to 50+ CU weighted to Olmsted Country
| SCI (N=70) | CU (N=350) | p value | |
|---|---|---|---|
| Age, yrs | |||
| Median (Q1, Q3) | 81 (76, 84) | 81 (76, 84) | 0.998* |
| Education, yrs | |||
| Median (Q1, Q3) | 15 (12, 18) | 14 (12, 17) | 0.104* |
| Sex | |||
| F=FEMALE | 30 (43%) | 150 (43%) | 1.000* |
| MCSA visit | |||
| Median (Q1, Q3) | 4 (3, 5) | 4 (3, 5) | 0.012* |
| MCSA visit grouped (12 3 4 5+) | |||
| Median (Q1, Q3) | 4 (3, 5) | 4 (3, 5) | 0.063* |
| CDR Sum of Boxes | |||
| Median (Q1, Q3) | 0.5 (0, 1.0) | 0 (0, 0) | < 0.001* |
| APOE ϵ4 carrier | |||
| 1=Yes | 23 (33%) | 83 (24%) | 0.108† |
| STMS, total | |||
| Median (Q1, Q3) | 33 (31, 34) | 36 (34, 37) | < 0.001* |
| Global, z | |||
| Median (Q1, Q3) | −0.42 (−0.81, −0.11) | 0.00 (−0.59, 0.66) | < 0.001* |
| Memory, z | |||
| Median (Q1, Q3) | −0.65 (−1.75, −0.08) | 0.23 (−0.44, 0.97) | < 0.001* |
| Attention, z | |||
| Median (Q1, Q3) | −0.40 (−0.79, 0.05) | −0.14 (−0.84, 0.33) | 0.581* |
| Language, z | |||
| Median (Q1, Q3) | −0.09 (−0.63, 0.26) | 0.00 (−0.55, 0.55) | 0.152* |
| Visuospatial, z | |||
| Median (Q1, Q3) | 0.20 (−0.68, 0.70) | 0.06 (−0.51, 0.69) | 0.538* |
| FAQ Total Score (0–30) | |||
| Median (Q1, Q3) | 0 (0,1) | 0 (0, 0) | < 0.001* |
Linear Model ANOVA
Pearson’s Chi-squared test
SCI = Subtle Cognitive Impairment; CU = cognitively unimpaired; IQR = interquartile range; CDR = Clinical Dementia Rating scale; STMS = Short Test of Mental Status; FAQ = Functional Activities Questionnaire
3.5. AUROC analyses
We then calculated the area under the receiver operating characteristic curve (AUROC) for the SCI cluster vs. the matched CU group and tested whether it was significantly different from 0 at the p=0.05 level. The most striking difference between the groups was in the memory z-score (AUROC 0.76; p=<.001) indicating that 76% of the time, a participant in the SCI cluster performed worse than their matched CU peer. There was also a significant difference on the global z-score (AUROC 0.67; p=<.001), indicating that 67% of the time, a participant in the SCI cluster performed worse than their matched CU peer. There were no differences in the language z (AUROC 0.56; p=.13), attention z (AUROC 0.55; p = .21) or visuospatial z (AUROC; 0.50 p = .58) scores.
3.6. Cox Proportional Hazards
Table 2 shows the percentage of individuals receiving a diagnosis of MCI at a future visit and hazard ratios. Of the 4592 participants who entered the study as CU, the probability of being classified as incident MCI/dementia was 15%. The absolute probabilities of receiving a second MCI/dementia diagnosis in the following 15 – 30 months after the initial MCI/dementia diagnosis for participants in the amnestic, dysnomic, and dysexecutive clusters were 60%, 49%, and 53%, respectively. For participants in the SCI cluster, the absolute probability of being classified as MCI/dementia at a future visit was 31%. The SCI cluster had an approximate 2-fold increased risk of a subsequent diagnosis of MCI/dementia while the amnestic, dysnomic, and dysexecutive clusters had an approximate 6-fold increased risk of a subsequent diagnosis of MCI/dementia. Comparisons of hazard ratios between groups are provided in Table 3. The hazard ratios differed between the SCI and MCI clusters but not among the MCI clusters. Including APOE carrier status in the model did not impact results in a qualitative manner.
Table 2.
Count (%) testing as MCI at a future visit and hazard ratio
| Group | N (%) | HR (95% CI) | P-value |
|---|---|---|---|
| CU (reference group) | 680 (15%) | 1.0 | <0.001 |
| SCU | 22 (31%) | 2.1 (1.3,3.2) | 0.001 |
| Amnestic | 115 (60%) | 6.2 (5.1,7.8) | <0.001 |
| Dysnomic | 41 (49%) | 6.0 (4.3, 8.2) | <0.001 |
| Dysexecutive | 83 (53%) | 6.1 (5.0,7.4) | <0.001 |
P-value from a cox-proportional hazards model; CU = cognitively unimpaired; SCI = subtle cognitive impairment
Table 3.
Comparisons of hazard ratios between groups
| SCI | Amnestic | Dysnomic | Dysexecutive | |
|---|---|---|---|---|
| CU | 0.001 | <0.001 | <0.001 | <0.001 |
| SCI | <0.001 | <0.001 | <0.001 | |
| Amnestic | 0.90 | 0.86 | ||
| Dysnomic | >0.99 | |||
CU = cognitively unimpaired; SCI = subtle cognitive impairment
4. Discussion
In this prospective, population-based, longitudinal study of participants with incident MCI (1) we empirically identified three distinct neuropsychological subtypes (amnestic, dysexecutive, dysnomic) as well as a fourth group with SCI that differed from those who remained CU; (2) participants in the SCI cluster had lower memory and global z-scores relative their robustly matched CU peers; (3) participants in the SCI cluster had an increased probability of progressing to MCI or dementia relative to their matched CU peers; and (4) the three distinct neuropsychological subtypes (amnestic, dysexecutive, dysnomic) share features with the subtypes identified by Edmonds et al. in the ADNI dataset.[13]
We used two methods to validate that the SCI cluster does not represent a group of false-positive participants. The first was an ROC curve analysis which used a robust matching procedure wherein we matched the participants with SCI to CU participants by age, sex, and number of exposures to previous neuropsychological tests given the known effects of previous test exposure on performance [35, 36]. Results revealed that participants in the SCI cluster performed more poorly than their matched CU peer 76% of the time on the memory z-score and 67% of the time on the global z-score. Furthermore, results from Cox Proportional Hazards revealed that the SCI cluster had a slightly greater than 2-fold increased probability of progression to MCI/dementia compared to the CU group.
Our group previously showed that even when a neuropsychological domain cut score of z = −0.5 was used (which is slightly greater than the memory z-scores of the SCI cluster with median = −0.6), there was increased risk of incident dementia. The incidence rates at this cut score were very low, but for multi-domain patterns, the hazard ratios were significant [37]. We also previously showed that a group of participants that developed incident MCI/dementia had lower baseline scores in all cognitive domains relative to those who remained CU, and the memory domain z-score at baseline of participants with incident MCI/dementia was over a half-standard deviation lower than the group that remained CU (i.e., a z-score difference of .72) [36]
Several studies show that individuals with MCI who revert to normal have an increased risk for receiving another MCI classification or developing dementia [38–43], and the reversion rates are higher in community-based samples [38, 41, 43]. Although participants in the SCI cluster had a higher rate of reversion to CU than participants in the other three clusters (70% versus 30% reversion rate), they were much more likely to receive a classification of MCI at the following visit than participants in the CU group. Because of inherent day to day variability in test-taking performance, the performance of persons with impending MCI may fluctuate in a range that straddles the cut-point between CU and MCI [39, 44]. The observation of reversion to CU does not invalidate the concept of MCI but rather reflects an inherent clinical feature of incident MCI due to variability in the participants’ ability to benefit from previous exposure to the testing [36], transient and/or reversible conditions present on the day of the evaluation, the informant’s perception of the participant, and interactions between the participant and clinicians [39]. Those individuals who revert to CU may already have some degree of underlying brain pathology given that individuals with MCI, including those who revert to CU, have a higher risk of progressing to dementia than those who have never received a diagnosis of MCI [38–42].
The amnestic, dysexecutive, and dysnomic clusters we identified in the MCSA dataset have some similarities and differences relative to those derived from the ADNI cohort [13] aside from the SCI cluster. Both the ADNI and MCSA datasets resulted in a cluster with isolated memory impairment. The MCSA dysexecutive cluster had relatively mild impairment in memory, language, and visuospatial function in addition to the prominent attention/executive impairment while in the ADNI dysexecutive cluster, memory was mildly impaired but language was substantially impaired. The MCSA dysnomic cluster had mild to moderate impairment in the memory, attention/executive, and visuospatial domains in addition to language whereas the ADNI dysnomic cluster also had impairment in memory but not attention or executive function. This could also be due to slight differences in the neuropsychological tests used to derive the clusters. Nonetheless, the empirical identification of amnestic, dysexecutive, and dysnomic clusters in these two large datasets provides support for reproducible MCI subtypes that when accounted for in clinical trials may uncover stronger relationships among biomarkers, pathology and outcomes, thus improving trial efficiency. Longitudinal evaluation of participants in these clusters will also provide additional insight into the clinical phenotypes of these groups.
Unlike the studies using the ADNI dataset, we did not identify a group of “cluster-derived normal” participants [11, 13]. There are several important methodological differences between the study by Edmonds et al [13] on the ADNI data and the MCSA that may shed light on why the participants in the SCI cluster in our study indeed likely represent early MCI rather than false positives. (1) We examined only participants diagnosed with incident MCI based on prospective ratings blinded to previous diagnosis whereas the ADNI participants were prevalent MCI diagnosed at their initial screening evaluation. (2) ADNI determines abnormal memory function based on a single memory score (i.e., delayed recall of story A from WMS-R Logical Memory with cut-offs that are education but not age-corrected). As explained by Edmonds et al, the use of only one memory measure to identify memory impairment is a possible shortcoming that could account for low specificity and the large number of false-positive classifications [12]. In contrast, the MCSA uses a composite score based on three age-adjusted measures [27] to assess memory (AVLT Delayed Recall, WMS-R Logical Memory II (both paragraphs), and WMS-R Visual Reproduction II); (3) ADNI assesses general cognitive function with only the MMSE whereas the MCSA uses the Short Test of Mental Status [15] and performance on neuropsychological measures of language, attention/executive, visuospatial function, and memory when determining cognitive status; (4) ADNI recruits participants from universities and medical centers [12] whereas the MCSA is an epidemiologic community-based sample [14]. (5) Finally, ADNI includes only participants with amnestic MCI who must have a CDR of 0.5 to enter the study. The MCSA, being population-based, did not have any restrictions on entry.
Somewhat unexpectedly, we did not identify a cluster with predominant visuospatial impairment despite including two measures that assess this domain, though both the dysexecutive cluster (visuospatial mean z = –1.34) and the dysnomic cluster (visuospatial mean z = −1.83) had below average to mildly impaired visuospatial z-scores. In a previous paper that examined prognosis in elderly persons without dementia in both the MCSA and Framingham Heart Study, the lowest rates of incident dementia occurred with the single-domain nonamnestic profile in the visuospatial domain while single-domain non-amnestic attention/executive function had a comparable prognosis to the single-domain amnestic profile, suggesting that visuospatial function does not add as much to prognosis as attention/executive function and memory [37]. Conversely, Clark et al [9] identified a visuospatial/visual memory subgroup identified using Comprehensive (vs. Conventional) MCI criteria that was characterized by lower performance [(z = −1.0 (1.6)] only on Block Design which they speculated might represent an emerging non-AD dementia or AD-related condition such as Dementia with Lewy Bodies.
In addition to identifying empirical MCI subtypes in the MCSA, our results underscore the value of identifying the earliest stage at which an individual begins to show evidence for cognitive decline, even if this decline does not yet meet a clinical threshold. We used the first diagnosis of MCI or dementia which allowed us to capture participants just as they were transitioning from a classification of CU to MCI. Although participants in the SCI cluster had a lower probability of being classified as MCI/dementia at a subsequent visit relative to the other MCI groups, they had a higher probability (i.e., double) compared to cognitively unimpaired participants. The characteristics of the SCI cluster may represent the transitional cognitive decline of Stage 2 of the new NIA-AA AD Research Framework [2] and thus could be a group to target with early interventions given they are showing the earliest manifestations of cognitive decline.
Strengths of our study include a large sample of participants from a population-based design and in-depth characterization including neuropsychological evaluation of four cognitive domains, information from an informant, a physician examination, and diagnosis made by a consensus process. Our ability to identify a separate cluster with SCI from the amnestic cluster underscores the importance of a thorough examination of memory and not relying solely on a single memory measure or preset cut-off score, subjective cognitive complaints, or subjective rating scales for identifying MCI. Participants were assessed at multiple time points, and at each assessment the raters did not know participants’ previous classification or the other raters’ classification. We also used a rigorous matching procedure for identifying CU participants against which to compare our SCI cluster. A limitation of this study is that our participants may be healthier than non-participants based on their ability to remain active in the MCSA for several years.
A future direction of our work will be to examine imaging biomarkers of our empirically derived MCI clusters to better understand the underlying pathophysiology, especially the group with subtle cognitive impairment. Specifically, the next step of our work will be to examine differences in cortical thickness in each of the cluster-derived incident MCI subtypes. Based on a previous study by Edmonds et al. [45] we predict that the amnestic and dysnomic clusters will have atrophy relatively restricted to the temporal lobe while the dysexecutive cluster will have atrophy in temporal, frontal, and parietal regions. Given that our SCI cluster has very mild memory impairment, we expect that this group will also have temporal lobe atrophy, albeit less extensive than the amnestic cluster.
RESEARCH IN CONTEXT.
Systematic review: We reviewed the literature in PubMed that focused on empirical methods for classifying mild cognitive impairment (MCI) subtypes based on conventional versus comprehensive criteria and the oversensitivity of conventional criteria that may result in misclassification of individuals as having MCI. However, these studies are based on prevalent MCI, and impairment in some cognitive domains may have progressed more than others.
Interpretation: The incident MCI cluster subtypes identified in the MCSA share some similarities and differences with those derived from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort, with the most notable difference being a cluster-derived normal group in ADNI vs. a group with subtle cognitive decline in the MCSA that differed from a matched cognitively unimpaired group.
Future directions: The identification of replicable MCI subtypes as well as individuals with subtle cognitive decline may allow for more precision in characterizing groups to target with early interventions.
Acknowledgments
The authors wish to thank the participants and staff at the Mayo Clinic Study of Aging. This research was made possible by the Rochester Epidemiology Project (R01 AG034676) and was supported by NIH grants R01 AG49810, P50 AG016574, U01 AG006786, and R01 AG041851, by the Robert Wood Johnson Foundation, The Elsie and Marvin Dekelboum Family Foundation, and by the Mayo Foundation for Education and Research. There are no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sperling RA, et al. , Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia, 2011. 7(3): p. 280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack CR Jr, et al. , NIA-AA Research Framework: Towards a biological definition of Alzheimer’s Disease. Alzheimer’s & Dementia, 2018. 14: p. 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delano-Wood L, et al. , Heterogeneity in mild cognitive impairment: Differences in neuropsychological profile and associate white matter lesion pathology. Journal of the International Neuropsychological Society, 2009. 15(6): p. 906–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libon DJ, et al. , The heterogeneity of mild cognitive impairment: a neuropsychological analysis. Journal of the International Neuropsychological Society, 2010. 16(1): p. 84–93. [DOI] [PubMed] [Google Scholar]
- 5.Folstein MF, Folstein SE, and McHugh PR, “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res, 1975. 12(3): p. 189–98. [DOI] [PubMed] [Google Scholar]
- 6.Winblad B, et al. , Mild cognitive impairment – beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine, 2004. 256(3): p. 240–246. [DOI] [PubMed] [Google Scholar]
- 7.Petersen RC, Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine, 2004. 256(3): p. 183–194. [DOI] [PubMed] [Google Scholar]
- 8.Damian M, et al. , Single-Domain Amnestic Mild Cognitive Impairment Identified by Cluster Analysis Predicts Alzheimer’s Disease in the European Prospective DESCRIPA Study. Dementia and Geriatric Cognitive Disorders, 2013. 36(1–2): p. 1–19. [DOI] [PubMed] [Google Scholar]
- 9.Clark LR, et al. , Are empirically-derived subtypes of mild cognitive impairment consistent with conventional subtypes? Journal of the International Neuropsychological Society, 2013. 19: p. 636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jak AJ, et al. , Quantificiation of five neuropsychological approaches to defining mild cognitive impairment. American Journal of Geriatric Psychiatry, 2009. 17(5): p. 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bondi MW, et al. , Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. Journal of Alzheimer’s Disease, 2014. 42(1): p. 275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen RC, et al. , Alzheimer’s Disease Neuroimaging Initiative (ADNI): Clinical characterization. Neurology, 2010. 74(3): p. 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edmonds EC, et al. , Susceptibility of the conventional criteria for mild cognitive impairment to false-positive diagnostic errors. Alzheimer’s & Dementia, 2015. 11(4): p. 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts RO, et al. , The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology, 2008. 30(1): p. 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kokmen E, et al. , The short test of mental status. Correlations with standardized psychometric testing. Arch Neurol, 1991. 48(7): p. 725–8. [DOI] [PubMed] [Google Scholar]
- 16.Blessed G, Tomlinson B, and Roth M, The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. British Journal of Psychiatry, 1968. 114: p. 797–811. [DOI] [PubMed] [Google Scholar]
- 17.Morris JC, The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology, 1993. 43(11): p. 2412–4. [DOI] [PubMed] [Google Scholar]
- 18.Pfeffer RI, et al. , Measurement of functional activities in older adults in the community. J Gerontol, 1982. 37(3): p. 323–9. [DOI] [PubMed] [Google Scholar]
- 19.Rey A, L’examen clinique en psychologie. 1964, Presses Universitaires de France: Paris. [Google Scholar]
- 20.Wechsler D, Wechsler Memory Scale-Revised. 1987, New York: The Psychological Corporation. [Google Scholar]
- 21.Kaplan E, Goodglass H, and Weintraub S, The Boston Naming Test. 1983, Philadelphia: Lea & Febiger. [Google Scholar]
- 22.Strauss E, Sherman EMS, and Spreen O, A Compendium of Neuropsychological Tests. 2006. New York: Oxford University Press. [Google Scholar]
- 23.Reitan R, Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual & Motor Skills, 1958. 8: p. 271–276. [Google Scholar]
- 24.Wechsler D, Wechsler Adult Intelligence Scale-Revised. 1981, San Antonio, TX.: The Psychololgical Corporation. [Google Scholar]
- 25.Ivnik RJ, et al. , Neuropsychological tests’ norms above age 55: COWAT, BNT, MAE Token, WRAT-R Reading, AMNART, Stroop, TMT, and JLO The Clinical Neuropsychologist, 1996. 10(3): p. 262–278. [Google Scholar]
- 26.Ivnik RJ, et al. , Mayo’s Older Americans Normative Studies: Updated AVLT norms for ages 56 to 97. The Clinical Neuropsychologist, 1992. 6((Supplement)): p. 83–104. [Google Scholar]
- 27.Ivnik RJ, et al. , Mayo’s Older Americans Normative Studies: WAIS-R, WMS-R and AVLT norms for ages 56 through 97. Clin Neuropsychol, 1992. 6(Supplement): p. 1–104. [Google Scholar]
- 28.Petersen RC, et al. , Practice guideline update summary: Mild cognitive impairment. Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology, 2018. 90(3): p. 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 4th ed. 1994, Washington, D.C.: American Psychiatric Association. [Google Scholar]
- 30.Hixson J and Vernier D, Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with Hhal. Journal of Lipid Research, 1990. 31(3): p. 545–548. [PubMed] [Google Scholar]
- 31.Kaufman L and Rousseeuw PJ, Finding Groups in Data: An Introduction to Cluster Analysis. 1990, New York, NY: Wiley. [Google Scholar]
- 32.Venables WN and Ripley BD, Modern Applied Statistics. 4th ed. 2002: Springer. [Google Scholar]
- 33.Acion L, et al. , Probabilistic index: an intuitive non parametric approach to measuring the size of treatment effects. Statistics in Medicine, 2006. 25(4): p. 591–602. [DOI] [PubMed] [Google Scholar]
- 34.Newcombe RG, Confidence intervals for an effect size measure based on the Mann–Whitney statistic. Part 2: asymptotic methods and evaluation. Statistics in Medicine, 2006. 25(4): p. 559–573. [DOI] [PubMed] [Google Scholar]
- 35.Ivnik RJ, et al. , Testing normal older people three or four times at 1- to 2-year intervals: defining normal variance. Neuropsychology, 1999. 13(1): p. 121–7. [DOI] [PubMed] [Google Scholar]
- 36.Machulda MM, et al. , Practice effects and longitudinal cognitive change in normal aging vs. incident mild cognitive impairment and dementia in the Mayo Clinic Study of Aging. The Clinical Neuropsychologist, 2013. 27(8): p. 1247–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knopman DS, et al. , Spectrum of cognition short of dementia. Framingham Heart Study and Mayo Clinic Study of Aging, 2015. 85(19): p. 1712–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malek-Ahmadi M, Reversion From Mild Cognitive Impairment to Normal Cognition: A Meta-Analysis. Alzheimer Disease & Associated Disorders, 2016. 30(4): p. 324–330. [DOI] [PubMed] [Google Scholar]
- 39.Roberts RO, et al. , Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology, 2014. 82(4): p. 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koepsell TD and Monsell SE, Reversion from mild cognitive impairment to normal or near-normal cognition: Risk factors and prognosis. Neurology, 2012. 79(15): p. 1591–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aerts L, et al. , Effects of MCI subtype and reversion on progression to dementia in a community sample. Neurology, 2017. 88(23): p. 2225–2232. [DOI] [PubMed] [Google Scholar]
- 42.Gao S, et al. , Mild cognitive impairment, incidence, progression, and reversion: findings from a community-based cohort of elderly African Americans. The American Journal of Geriatric Psychiatry, 2014. 22(7): p. 670–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Canevelli M, et al. , Spontaneous Reversion of Mild Cognitive Impairment to Normal Cognition: A Systematic Review of Literature and Meta-Analysis. Journal of the American Medical Directors Association, 2016. 17(10): p. 943–948. [DOI] [PubMed] [Google Scholar]
- 44.Lopez OL, et al. , Incidence of mild cognitive impairment in the Pittsburgh Cardiovascular Health Study–Cognition Study. Neurology, 2012. 79(15): p. 1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edmonds EC, et al. , Heterogeneous cortical atrophy patterns in MCI not captured by conventional diagnostic criteria. Neurology, 2016. 87(20): p. 2108–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]