Abstract
Purpose:
To determine whether preoperative endothelial cell density (ECD) and/or postoperative ECD after Descemet stripping automated endothelial keratoplasty (DSAEK) are associated with late endothelial graft failure (LEGF) in the Cornea Preservation Time Study (CPTS).
Design:
Cohort study within multicenter randomized clinical trial.
Participants:
1,007 individuals (1,223 study eyes), mean age 70 years, undergoing DSAEK for Fuchs’ dystrophy (94% of eyes) or pseudophakic/aphakic corneal edema (PACE) (6% of eyes) and followed for up to 5 years.
Methods:
Central ECD was determined by a central image analysis reading center. Preoperative ECD was determined for 1209 eyes that did not fail and 14 eyes that experienced LEGF. ECD at 6 and 12 months after DSAEK, the change in ECD from preoperative to 6 and 12 months, surgeon-reported operative complications, and postoperative graft dislocation, were investigated for an association with LEGFs unrelated to other postoperative events. Univariable and multivariable Cox proportional hazards regression models were used to assess associations.
Results:
The cumulative probability of LEGF was 1.3% (95% CI 0.8% to 2.4%). Median (IQR) preoperative ECDs were similar for eyes with LEGF (2523 (2367, 3161) cells/mm2) and eyes without failure (2727 (2508, 2973) cells/mm2) (p=0.34). ECD at 6 months was associated with LEGF (p<0.001) in time-to-event analyses, whereas preoperative ECD was not (p=0.55). The cumulative incidence (95% CI) of LEGF was 6.5% (3.0%, 14.0%) for 97 grafts with a 6 month ECD less than 1200 cells/mm2, 0.3% (0.0%, 2.4%) for 310 grafts with a 6 month ECD between 1200 and 2000 cells/mm2, and 0.6% (0.1%, 2.7%) for 589 grafts with a 6 month ECD greater than 2000 cells/mm2. In multivariable analyses, ECD at 6 months and operative complications were both associated with LEGF (p=0.002 and p=0.01, respectively), whereas graft dislocation was not (p=0.61).
Conclusions:
In eyes undergoing DSAEK, preoperative ECD is unrelated to LEGF, whereas lower ECD at 6 months is associated with LEGF. Early endothelial cell loss after DSAEK and intraoperative complications should be minimized to improve graft survival.
Precis
In the Cornea Preservation Time Study, preoperative endothelial cell density was not associated with late endothelial graft failure, whereas intraoperative complications and lower endothelial cell density at 6 months were associated with it.
Introduction
In a subset of participants of the Cornea Donor Study (CDS)1,2 who were included in the Specular Microscopy Ancillary Study (SMAS),3,4 lower endothelial cell density (ECD) at 6 months after penetrating keratoplasty (PK) was associated with a higher risk of late endothelial graft failure (LEGF) over 5 years. The SMAS analysis assessed LEGF not due to rejection or trauma, and also found that preoperative donor ECD was not predictive of such failure.5 PK was the technique of choice for corneal endothelial failure when participants were recruited to the CDS, but endothelial keratoplasty has since become the standard of care and most common surgical approach for this indication in the United States.6 Given differences in donor preparation, surgical manipulation, and changes in postoperative ECD over time between PK and Descemet stripping automated endothelial keratoplasty (DSAEK),7,8 we could not assume that the relationship between preoperative and/or postoperative ECD and LEGF after DSAEK was the same as that after PK.
To investigate this relationship in the context of DSAEK, we identified cases of LEGF unrelated to other causes (e.g., immunologic event, glaucoma, infection, or traumatic event) in the Cornea Preservation Time Study (CPTS).9 The CPTS was designed to determine the effect of preservation time (PT) on graft survival after DSAEK, and found that preservation time (PT) <12 days had little effect on graft survival at 3 years.10 The overall graft survival rate was 94%. The endothelial cell loss (ECL) results paralleled the graft success findings with no difference up to 13 days of PT.11 ECL was similar for the two groups at 37% for corneas preserved for 0–7 days and 40% for corneas preserved for 8–14 days.
In this study, we specifically assessed differences in ECD between corneas that experienced LEGF and those that were still surviving at the end of follow-up of up to 5 years. The goal of this analysis was to determine if there was an association between preoperative or postoperative ECD and LEGF in the CPTS.
Methods
The CPTS protocol and primary results have been described in detail previously,9–11 and pertinent aspects are described here. In this multicenter study that included 40 sites and 70 surgeons, patients undergoing DSAEK were randomized to receive a donor cornea preserved from 0–7 days versus 8–14 days. All participants provided written informed consent for follow-up through 3 years as that was the primary outcome assessment time-point for overall graft success. Participants that provided written informed consent for extended follow-up were additionally followed for up to 5 years. The protocol was approved by institutional review boards overseeing each site and adhered to the tenets of the Declaration of Helsinki. The study was registered as a clinical trial at www.clinicaltrials.gov (NCT 01537393).
Participants were enrolled between April 2012 and February 2014, were 42 to 90 years old (median, 70 years) at enrollment, and had corneal endothelial dysfunction requiring DSAEK (Fuchs’ endothelial corneal dystrophy [FECD], 94% of eyes; or pseudophakic or aphakic corneal edema [PACE], 6% of eyes). To minimize any confounding factor of glaucoma on the association of PT on graft success and endothelial cell loss, only eyes with intraocular pressure under 25 mmHg with or without glaucoma medication were enrolled. Eyes with trabeculectomy were eligible (7 of the 1330 eyes), but eyes with tube shunts were excluded even if intraocular pressure was under 25 mmHg. Donor age was 12 to 75 years old (median, 61 years); donor corneas had a minimum eye bank-measured central ECD of 2300 cells/mm2 and were stored at 2°C - 8°C in either Optisol GS (Bausch & Lomb, Rochester, NY) or Life 4°C (Numedis, Inc., Minneapolis, MN). Donor, recipient, operative, and postoperative variables were tracked prospectively.9 Operative complications were reported by the surgeons, and included pre-defined categories of inversion of the graft (also described as donor lenticule in our previous publication12), difficulty unfolding and positioning the graft with or without direct contact with the graft, difficulty managing the air bubble and its retention, and reinsertion of the graft after extrusion;12 they were also allowed to write-in other unusual observations at the time of surgery. The write-in observations were reviewed by the CPTS Study Chair (JL) and either assigned to one of the pre-defined categories, listed as an “other complication”, or deleted as an irrelevant operative complication (one subconjunctival hemorrhage). Postoperative factors included air injections and other donor repositioning maneuvers to manage graft malpositions including graft dislocation. Graft dislocation was defined as total detachment of the graft or graft that was attached but decentered requiring a surgically induced detachment, repositioning, and reattachment with air injection.
Endothelial imaging and image analysis
Endothelial photographs of the donor cornea were acquired by each certified eye bank before DSAEK using specular microscopy. After donor cornea procurement and before randomization, eye banks obtained one to three initial specular image(s) of the central endothelium after warming the donor tissue to room temperature, and then determined ECD by their usual analysis method (referred to as “screening” ECD). This ECD determination was utilized to qualify for donor entry into the CPTS with a minimum of 2300 cells/mm2. If a donor cornea was assigned to the CPTS, eye banks also obtained three images of the central endothelium either after lamellar dissection by the eye bank for preparation of the lenticule, or, if the donor cornea was to be prepared by the surgeon, prior to shipment (“preoperative” ECD). Donor corneas were again allowed to warm to room temperature to obtain the best image quality.13,14 After DSAEK, three images of the central donor corneal endothelium were obtained by each certified clinical site using specular or confocal microscopy at 6 months, 1 year and then annually as long as the graft survived.
All preoperative endothelial images were transferred to the Corneal Image Analysis Reading Center (CIARC) at Case Western Reserve University and University Hospitals Eye Institute (Cleveland, OH) by the Data Management and Analysis Center (DMAC, Jaeb Center for Health Research, Tampa, FL) in a de-identified manner. The CIARC served as the image analysis reading center and was responsible for quality control measures at the eye banks and clinical sites, details of which have been described previously.9,11,15,16 ECD was measured by two independent and masked observers using a variable frame analysis with adjudication procedures if necessary.15 Since the eye banks were not required by protocol to transfer screening images to the CIARC for image analysis, an incomplete set of screening images (75%) were available for CIARC analysis. Consequently, this report is based on the preoperative and postoperative image sets which were nearly all available for analysis by CIARC for central donor ECD determination. Although the ECD determined by the eye bank at the time of screening was restricted to be >2300 cells/mm2 for the donor cornea to be eligible for placement in the CPTS similar to the CDS4, there was no restriction on the preoperative ECD as determined by the CIARC. Among all of the eyes with ECD determined by the CIARC, 113 (9%) had ECD <2300 cells/mm2. Of these 113 eyes, 100 were between 2000 to 2299 cells/mm2, and only 13 below 2000 cells/mm2.
Late Endothelial Graft Failure
LEGF was defined as a graft that initially resulted in a clear recipient stroma in the first 8 week postoperatively that subsequently became cloudy or equivocally cloudy for at least 90 days, or that required regrafting due to presumed endothelial cell attrition unrelated to any immunologic (graft rejection) or acute event that might be deleterious to the endothelium.5 That is, primary donor and early failures (which in both instances did not clear or were replaced within 8 weeks) were excluded from all analyses of LEGFs.9,12 All eyes that failed due to a cause other than LEGF also were excluded. Eyes were censored if they had acute events after DSAEK defined as: air release due to pupillary block with high intraocular pressure, new onset angle closure, intraocular pressure >40, blunt and/or penetrating trauma, incisional or laser glaucoma surgery, any type of intraocular lens manipulation (except YAG capsulotomy), any incisional or laser iris surgery, or vitreous tap or YAG vitreolysis. Of note, secondary air injection procedures with or without graft repositioning after DSAEK were not censored and were included as a common event following primary surgery (10% of eyes needed at least one secondary air injection in the CPTS).10
Statistical Analysis
The cumulative incidence of LEGF was estimated by the Kaplan-Meier method and differences among subgroups were assessed with the log rank statistic. In all analyses, time was categorized based on intervals corresponding to the study examinations required by the protocol. Data were censored at the time of a definite graft rejection episode, an acute event that could affect the endothelium (as defined above), or the time of the last visit. Analyses including ECD at 6 months or ECD at 12 months excluded all data prior to the time of the follow-up ECD determination.
The association of preoperative ECD, postoperative ECD, change from baseline in ECD at various time points, and operative complications with LEGF was assessed using Cox proportional hazards regression models. Hazard ratios (HR) were reported for a decrease in ECD of 500 cells/mm2. The discrete logistic model option was applied to all Cox models. In addition to univariable models, models including ECD at 6 months and one other variable (either operative complications, graft dislocation, or donor history of diabetes) were evaluated to assess the association with LEGF when the two variables were considered simultaneously. Additionally, linear regression models were used to test for an association between 6 month ECD from operative complications, graft dislocation, and donor history of diabetes. Spearman correlation coefficients were used to assess the association between postoperative ECD and change from preoperative ECD at 6 and 12 months.
Preoperative ECD, and ECD at 6 and 12 months among eyes with LEGF were compared between the SMAS (for PK)5 and the CPTS (for DSAEK) by using two sample t-tests assuming equal variances in each group. Two sample t-tests were also used to compare preoperative ECD and ECD at 6 and 12 months between the SMAS and the CPTS among non-failures with an available preoperative measurement. From the CPTS data, Wilcoxon tests were used to compare preoperative ECD, ECD at 6 months, and ECD at 12 months between eyes that failed due to LEGF and those that did not.
All p-values were two-sided, and p<0.05 was considered statistically significant. No adjustments were made for multiple comparisons. All statistical analyses were made using SAS, version 9.4 (SAS Inc).
Results
Of the 1,330 donors, 1,313 preoperative image sets were submitted to CIARC, while 17 (1.0%) were not available for analysis. Only 27 of the 1,313 preoperative image sets (2%) were deemed unanalyzable by the CIARC. This resulted in 1286 available preoperative image sets. Additionally, all failures due to causes other than endothelial decompensation were excluded, leaving 1223 eyes to be included in analyses for preoperative ECD. These 1223 eyes comprised 1007 DSAEK recipients and were similar to the overall CPTS cohort; recipient age was 70 ± 9 years (mean ±SD), 616 (61%) subjects were female, 912 (91%) were white, and 944 (94%) had FECD versus 63 (6%) with PACE.
ECD over time in the late endothelial graft failures
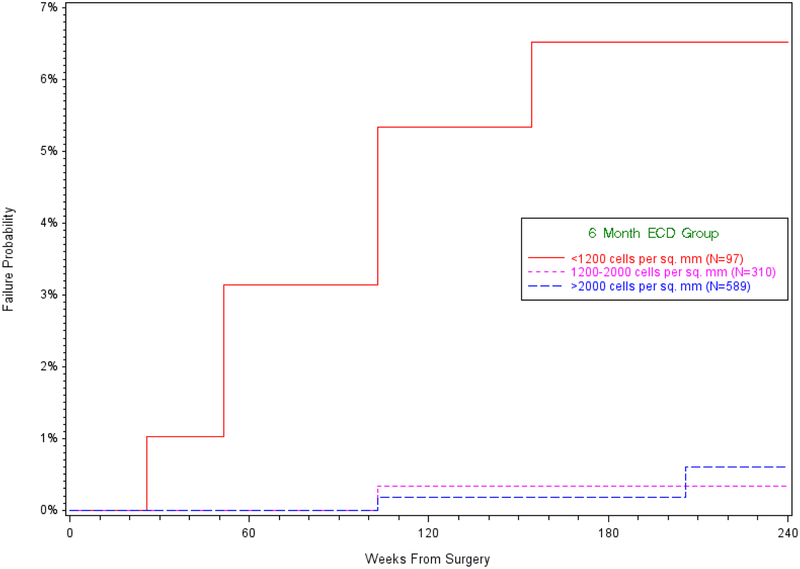
LEGF occurred in 14 grafts, with 2 failures by 6 months, 3 failures between 6 and 12 months, 3 failures between 12 and 24 months, 5 failures between 24 and 36 months, and 1 failure between 36 and 48 months (Figure 1). The cumulative probability of LEGF was 1.3% (95% CI 0.8% to 2.4%). Median (IQR) preoperative ECD was 2523 cells/mm2 (2367, 3161) in these 14 grafts and 2727 cells/mm2 (2508, 2973) in the 1209 grafts that survived (p=0.34). Among eyes with both preoperative and 6-month ECD measurements available, median (IQR) ECD at 6 months was 1127 cells/mm2 (463, 1875) in the 9 grafts that subsequently failed and 2120 cells/mm2 (1728, 2476) in the 987 grafts that survived (p=0.005). Among eyes with both preoperative and 12-month ECD measurements, median (IQR) ECD at 12 months was 971 cells/mm2 (838, 1601) in the 4 grafts that subsequently failed and 2072 cells/mm2 (1630, 2411) in the 931 grafts that survived (p=0.03).
Figure 1.
Kaplan-Meier estimates of the cumulative incidence of late endothelial graft failure
Associations with late endothelial graft failure
The cumulative incidence (95% CI) of LEGF was 6.5% (3.0%, 14.0%) for grafts with a 6 month ECD less than 1200 cells/mm2 (N=97), 0.3% (0.0%, 2.4%) for grafts with a 6 month ECD between 1200 and 2000 cells/mm2 (N=310), and 0.6% (0.1%, 2.7%) for grafts with a 6 month ECD greater than 2000 cells/mm2 (N=589) (Figure 1; log rank p<0.001). Preoperative ECD was not associated with LEGF (p=0.55, Table 1). In univariable analyses, LEGF was associated with ECD at 6 and 12 months, and with the change from preoperative ECD to 6 and 12 months (Table 1). Postoperative ECD and the change from preoperative ECD were highly correlated at 6 months (r=0.76; p<0.001) and 12 months (r=0.78; p<0.001).
Table 1.
Proportional Hazards Regression Analyses for the Association of Endothelial Cell Density with Late Endothelial Graft Failure
| N | Hazard Ratio (95% CI) | Pa | |
|---|---|---|---|
| All Late Endothelial Graft Failures | |||
| Preoperative ECDb | 1223 | 1.25 (0.61, 2.57) | 0.55 |
| Late Endothelial Graft Failures after the 6 Month Visit | |||
| Preoperative ECDb | 966 | 1.37 (0.55, 3.43) | 0.50 |
| Change in ECD from Preoperative to 6 Monthsc | 966 | 2.09 (1.40, 3.12) | <0.001 |
| ECD at 6 Monthsb | 996 | 2.74 (1.60, 4.67) | <0.001 |
| Late Endothelial Graft Failures after the 12 Month Visit | |||
| Preoperative ECDb | 905 | 0.74 (0.21, 2.60) | 0.64 |
| Change in ECD from Preoperative to 12 Monthsc | 905 | 2.57 (1.31, 5.01) | 0.006 |
| ECD at 12 Monthsb | 935 | 2.95 (1.23, 7.05) | 0.02 |
Based on a univariable Cox model.
Per 500 cells/mm2 lower ECD
Per 500 cells/mm2 decrease from the preoperative value
ECD = endothelial cell density
Preoperative endothelial images were analyzed by the reading center after lamellar dissection to create the donor lenticule by eye bank or prior to shipping for surgeon-prepared tissue
LEGF was associated with operative complications (p=0.01) and ECD at 6 months (p=0.002) (Table 2). Specifically, the hazard ratio (95% CI) of a decrease in 500 cells/mm2 in ECD at 6 months for LEGF was 2.43 (1.40, 4.20) and the hazard ratio (95% CI) of having an operative complication (vs. not having an operative complication) was 6.47 (1.51, 27.75). Operative complications also were associated with ECD at 6 months (p<0.001), with mean ECD at 6 months being 401 cells/mm2 lower (95% CI; 221 to 581 cells/mm2) in eyes with operative complications compared to eyes without them. Among grafts with a 6 month ECD of ≤1200 cells/mm2, LEGF occurred in 3 of 12 (25%) eyes that had operative complications, and 3 of 85 (4%) eyes that did not. For grafts with a 6 month ECD >1200 cells/mm2, LEGF occurred in 0 of 30 (0%) eyes that had operative complications and 3 of 869 (0.3%) eyes that did not. The change in ECD and percent loss for each type of operative complication experienced among the 1223 eyes included in analyses for preoperative ECD are summarized in eTable 1.
Table 2.
Proportional Hazards Regression Analyses for Association of Endothelial Cell Density at 6 Months and Operative Complications with Late Endothelial Graft Failure
| N | Hazard Ratio (95% CI) | Pa | |
|---|---|---|---|
| Univariable Modelsb | |||
| ECD at 6 Monthsc | 996 | 2.74 (1.60, 4.67) | <0.001 |
| Operative Complications | 996 | 12.92 (3.20, 52.09) | <0.001 |
| Multivariable Model | |||
| ECD at 6 Monthsc | 996 | 2.43 (1.40, 4.20) | 0.002 |
| Operative Complications | 996 | 6.47 (1.51, 27.75) | 0.01 |
Based on a Cox model.
Includes 30 eyes that did not have a preoperative ECD measurement available.
Per 500 cells/mm2 lower ECD
ECD = endothelial cell density
Preoperative endothelial images were analyzed by the reading center after lamellar dissection to create the donor lenticule by eye bank or prior to shipping for surgeon-prepared tissue
eTable 1.
Change in ECD and Percent Loss by Type of Operative Complication Experienced during the CPTS
| Operative Complication | Total Number of Eyes | Total Number of Eyes Included at 6 Months | Change from Preoperative to 6 Month Postoperative ECD (cells/mm2, mean ± SD)b |
Percent Loss from Preoperative to 6 Month Postoperative ECD (mean ± SD)b |
Total Number of Eyes Included at 3 Years | Change from Preoperative to 3 Year Postoperative ECD (cells/mm2, mean ± SD)c |
Percent Loss from Preoperative to 3 Year Postoperative ECD (mean ± SD)c |
|---|---|---|---|---|---|---|---|
| Eyes without Operative Complications | 1161 | 924 | −691 ± 573 | 25% ± 20% | 703 | −1042±601 | 38% ± 21% |
| Eyes with Operative Complications | 62a | 42 | −1192 ± 798 | 41% ± 26% | 28 | −1561 ± 724 | 54% ± 24% |
| Difficult Unfolding and Positioning Graft with Use of Positioning Hook | 15 | 8 | −944 ± 662 | 33% ± 24% | 5 | −1642 ± 688 | 59% ± 26% |
| Difficult Unfolding and Positioning Graft without Use of Positioning Hook | 11 | 9 | −1153 ± 903 | 39% ± 27% | 6 | −1317±553 | 48% ± 22% |
| Difficult Air Fill and Retention in Positioning | 11 | 7 | −911 ±828 | 34% ± 32% | 3 | −1354±1134 | 45% ± 35% |
| Vitreous Loss | 7 | 3 | −987 ± 467 | 34% ±18% | 2 | −1405 ± 711 | 47% ± 23% |
| Posterior Capsule Rupture | 6 | 2 | −929 ± 750 | 35% ± 26% | 1 | −821 ± 0 | 34% ± 0% |
| Reinsertion of Graft after Extrusion | 4 | 3 | −2032 ± 247 | 72% ± 11% | 3 | −2026 ± 455 | 72% ± 12% |
| Significant Hyphema | 3 | 3 | −2014±939 | 63% ± 30% | 2 | −2456 ± 294 | 78% ± 4% |
| Inverted Graft | 2 | 2 | −1834±1248 | 56% ± 36% | 2 | −2656 ±11 | 83% ± 3% |
| Suprachoroidal Hemorrhage | 0 | 0 | NA | NA | 0 | NA | NA |
| Otherd | 13 | 10 | −1298 ± 675 | 42% ±18% | 8 | −1564 ± 803 | 53% ± 26% |
Some eyes experienced multiple types of operative complications and therefore the sum of specific operative complications was greater than 62.
Summary statistics were only calculated including eyes with an available ECD measurement at 6 months. There were 42 such eyes.
Summary statistics were only calculated including eyes with an available ECD measurement at 36 months. There were 28 such eyes.
Includes six eyes with a clouded donor stroma, one eye with elevated IOP with open angle, one eye with posterior pressure with iris prolapse, one eye with a post lamellar dissection thickness measurement issue, and four eyes with a donor preparation issue.
ECD = endothelial cell density
Notably nearly one-quarter of study eyes experienced an abnormal postoperative IOP event at least one month following DSAEK with no impact on graft success or ECD (data not shown). Eight eyes were censored for IOP elevations > 40 mmHg for this analysis. Among the 143 eyes that had a steroid-induced pressure elevation ≤ 40 mmHg, the rate of LEGF was similar to the overall cohort.
We explored the influence of other factors of interest on LEGF and ECD at 6 months. Graft dislocation was associated with ECD at 6 months (p<0.001), with mean ECD at 6 months being 703 cells/mm2 lower (95% CI; 547 to 858 cells/mm2) in eyes that had a graft dislocation compared to those that did not. After adjusting for ECD at 6 months, there was no statistically significant association of graft dislocation with LEGF (hazard ratio = 0.57, 95% CI (0.07, 4.89), p=0.61). Donor history of diabetes was associated with ECD at 6 months (p<0.001), with mean ECD at 6 months being 149 cells/mm2 lower (95% CI; 66 to 232 cells/mm2) in eyes with donors with diabetes compared to donors without diabetes. After adjusting for ECD at 6 months, there was no statistically significant association of donor history of diabetes with LEGF (hazard ratio = 0.99, 95% CI (0.24, 4.03), p=0.99).
Comparison of the SMAS and the CPTS associated factors for LEGF
Preoperative ECD did not differ for grafts with LEGF after DSAEK in the CPTS compared to after PK in the SMAS5 (p=0.83) (Table 3). Although there was a trend towards lower ECD at 6 and 12 months after DSAEK in the CPTS compared to the SMAS, the differences were not statistically significant (Table 3). Among non-failures, preoperative ECD was higher in the CPTS compared to the SMAS (p<0.001), whereas ECDs at 6 (p<0.001) and 12 (p<0.001) months were lower in the CPTS compared to the SMAS (Table 3).
Table 3.
Comparison of Mean ECD between the SMAS5 and the CPTS
| Late Endothelial Graft Failures | Non-Failures | |||||
|---|---|---|---|---|---|---|
| ECD, cells/mm2 (mean ± SD) |
SMAS (N=17) | CPTS (N=14) | P a | SMAS (N=483) | CPTS (N=1209) | Pa |
| Preoperative | 2671 ± 272 | 2703 ± 528 | 0.83 | 2684 ± 311 | 2759 ± 383 | <0.001 |
| 6 Monthsb | 1733 ± 801 | 1274 ± 888 | 0.27 | 2439 ± 575 | 2058 ± 581 | <0.001 |
| 12 Monthsc | 1500 ± 689 | 1219±612 | 0.48 | 2221 ±631 | 2003 ± 599 | <0.001 |
Two sample t-test assuming equal variances
Includes 9 eyes in the SMAS and 9 eyes in the CPTS that failed. Among non-failures, includes 266 eyes from the SMAS and 987 eyes from the CPTS.
Includes 12 eyes in the SMAS and 4 eyes in the CPTS that failed. Among non-failures, includes 330 eyes from the SMAS and 931 eyes from the CPTS.
ECD = endothelial cell density; SMAS = Specular Microscopy Ancillary Study; CPTS = Cornea Preservation Time Study
Discussion
Preoperative ECD was not associated with LEGF after DSAEK in the CPTS, whereas ECD at 6 months and intraoperative complications were each associated with LEGF. Intraoperative difficulties were therefore the main controllable factor affecting endothelial cell loss over the first 6 months. Although ECD at 12 months also was associated with LEGF, the 6-month ECD data were more robust because of the larger number of subsequent LEGFs. The CPTS results for LEGF after DSAEK parallel those from the donor corneas undergoing PK in the SMAS.5 Specifically, ECD at 6 months after PK, but not preoperative ECD, predicted subsequent LEGF in the SMAS.5 Nishimura and colleagues found that ECD at 2 months predicted LEGF after PK, although they also found that lower preoperative ECD increased the risk of LEGF as well.17 The study by Nishimura and colleagues included low-risk indications for graft failure, whereas the CPTS and the SMAS included moderate-risk indications for graft failure, which might explain the different results.
The results of this study are important because of distinct differences between DSAEK and PK that might have influenced the relationship between postoperative ECD and LEGF. These differences include donor tissue preparation, intraoperative manipulation of the graft, and variations in ECL over time.18,19 The impact of donor tissue preparation on ECL in the CPTS will be described in another report.20 After DSAEK, the largest decline in ECD occurs by the first month21 with a much lower and relatively linear rate of cell loss through several years thereafter,21 implicating surgical and very early postoperative trauma as major factors influencing ECL. In contrast, ECL after PK follows a bi-exponential pattern with a more gradual but sustained ECL over the first 5 years compared to DSAEK.4,7,18,22,23 These different patterns of ECL were evident between the CPTS and the SMAS, with ECD (among non-failure cases) being higher preoperatively and lower at 6 and 12 months in the CPTS (Table 3). Although the patterns of ECL are different between DSAEK and PK, that ECD at 6 months was associated with LEGF after both procedures indicates that early ECL from any cause should be minimized to improve graft survival.
In previous analyses, we found that ECL (at 3 years) was greater in eyes that had operative complications.20 Therefore, we controlled for the potential confounding effect of operative complications in the multivariable analysis of ECD on LEGFs and found that both operative complications and ECD were associated with increased risk when considered simultaneously. Difficulty with inserting, unfolding, or positioning DSAEK grafts typically requires more manipulation of the graft than usual and this was associated with increased ECL at 6 months (33–39%) and 3 years (45–59%) compared to eyes without operative complications (25% at 6 months, and 38% at 3 years) (eTable 1).19 The highest ECL was associated with graft extrusion, hyphema, and inverted grafts (eTable 1). Graft dislocation was associated with lower ECD at 6 months, similar to a previous study,24 but was not an independent risk factor for LEGF when modeled with ECD at 6 months (of note, graft dislocation was found to be a risk factor for early and primary failures in unpublished data from the CPTS). Thus, the occurrence of operative complications is the most important modifiable risk factor for reducing LEGF, and should be minimized to improve DSAEK graft survival. It is important to reiterate that even in the absence of operative complications, lower ECD at 6 months was also associated with LEGF, indicating that factors other than surgical trauma also influence early ECL and LEGF.
We previously reported no association between preoperative ECD and primary donor or early failures in the CPTS.12 The overall primary donor and early failure rate after DSAEK in the CPTS was 3.4% (45 grafts), and 33 of these failures were not associated with intraoperative complications.10 This is in contrast to the 0.3% primary donor failure rate after PK in the CDS,1 and indicates that manipulation of the donor tissue for DSAEK is much more traumatic than for PK, even if surgeons report their cases as uncomplicated. It is unlikely that true primary donor failure, caused by factors intrinsic to the donor tissue, is higher in DSAEK than PK. The 10-fold increase in early failure after DSAEK, even in uncomplicated cases, is most likely explained by the increased graft manipulation or decreased resilience of the donor endothelium to manipulation. The mechanism of failure in these cases is probably similar to that of LEGF, in that there is very high early ECL with insufficient residual endothelial function for the graft to clear after surgery. The latter is supported by the eyes with LEGF after DSAEK in the CPTS having a trend towards much lower ECD at 6 months than those after PK in the SMAS4 (Table 3). This also suggests that there is variability in how well individual corneal grafts tolerate or respond to surgical manipulation during or immediately after DSAEK. Diabetes in the donor was associated with lower ECD at 6 months, and this could be one factor that may influence this individual donor performance variability. The latter is supported by findings in the CPTS that a higher graft failure rate12 and lower ECD at 3 years20 was associated with diabetes in the donor.
The major strength of this study was its large, prospective design with careful determination of outcomes and ECD that enabled this secondary analysis to be performed. The results of this study, however, should be considered carefully given there were only 14 cases of LEGF unrelated to any acute or immunologic events. The limitations of the study included subgroup analyses by recipient diagnosis that could not be performed due to the small number of eyes with PACE and the small number of events. Similarly, analyses could not be performed separately for the different types of operative complications due to small numbers of eyes with any complication, and ECL associated with specific operative complications (eTable 1) should therefore be interpreted cautiously. Although screening ECD (measured by the eye bank using their standard procedure) had to be ≥2300 cells/mm2, the full range of preoperative ECDs determined by the CIARC were available for this analysis and this included 12% of the preoperative ECDs below 2300 cells/mm2. Even with this percentage below the 2300 cells/mm2, preoperative ECD was not associated with LEGF. We previously showed that a lower screening ECD was associated with a lower ECD at three years20 similar to a DMEK study with baseline ECD ≤2100 cells/mm2 reported a risk factor for a lower ECD under 1000 cells/mm2 at one year.25 Nevertheless, our preoperative ECD, following the determination of the screening ECD and reflecting the storage period and lamellar dissection processing, in the case of the eye-bank prepared donor tissue, did not associate with more LEGFs. Finally, longer follow-up with more LEGFs would have improved our statistical power for these analyses.
In summary, while appreciating these limitations we are confident with our finding that ECD at 6 months was associated with LEGF following DSAEK in the CPTS and consistent with results following PK from the SMAS.5 This finding reinforces that surgeons should recognize preoperative ECD as not correlated with LEGF after DSAEK, and that minimizing early ECL and operative complications are critical for improving graft survival. In addition, a primary donor and early failure rate of <3% was a selection criterion for surgeons to participate in the CPTS,10 and might not reflect the rate of individual surgeons as this can vary widely.26 Therefore it is important for all surgeons to determine their individual graft survival outcomes, and those with graft failure rates higher than in the CPTS should optimize their surgical technique to minimize cumulative early graft trauma and improve graft survival. Our finding that ECD at 6 months was independently associated with LEGF suggests that, in addition to reducing operative complications, eye bank research should explore whether endothelial cell resilience is influenced by factors such as diabetes in the donor to reduce the variability in ECL during normal and complicated surgical manipulation.
Acknowledgements
Financial Support and Role of Sponsors: Supported by cooperative agreements with the National Eye Institute, National Institutes of Health, Department of Health and Human Services (EY20797 and EY20798) which had roles in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication. The following sponsors had no role the design, conduct, management, analysis, interpretation or publication of the study data, but provided additional support for ancillary work pertaining to this study: Eye Bank Association of America, The Cornea Society, Vision Share, Inc., Alabama Eye Bank, Cleveland Eye Bank Foundation, Eversight, Eye Bank for Sight Restoration, Iowa Lions Eye Bank, Lions Eye Bank of Albany, San Diego Eye Bank, and SightLife.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The following authors have financial interests outside the submitted work with companies that manufacture corneal storage solutions (considered relevant to this work): Mark Terry (Bausch & Lomb), and W. Barry Lee (Bausch & Lomb). The financial interests were related to royalties for endothelial keratoplasty surgical instruments and educational grants (M. Terry) and speaker’s bureau fees (W.B. Lee).
References
- 1.Gal RL, Dontchev M, Beck RW, et al. The effect of donor age on corneal transplantation outcome results of the Cornea Donor Study. Ophthalmology. 2008;115:620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mannis MJ, Holland EJ, Gal RL, et al. The effect of donor age on penetrating keratoplasty for endothelial disease: graft survival after 10 years in the Cornea Donor Study. Ophthalmology. 2013;120:2419–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lass JH, Benetz BA, Gal RL, et al. Donor age and factors related to endothelial cell loss 10 years after penetrating keratoplasty: Specular Microscopy Ancillary Study. Ophthalmology. 2013;120:2428–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lass JH, Gal RL, Dontchev M, et al. Donor age and corneal endothelial cell loss 5 years after successful corneal transplantation. Specular Microscopy Ancillary Study results. Ophthalmology. 2008;115:627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lass JH, Sugar A, Benetz BA, et al. Endothelial cell density to predict endothelial graft failure after penetrating keratoplasty. Arch Ophthalmol. 2010;128:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eye Bank of America. 2017. Eye Banking Statistical Report. 2018, http://restoresight.org/wp-content/uploads/2016/03/2015-Statistical-Report.pdf. Accesses August 2, 2017. [Google Scholar]
- 7.Price MO, Calhoun P, Kollman C, et al. Descemet stripping endothelial keratoplasty: Ten-year endothelial cell loss compared with penetrating keratoplasty. Ophthalmology. 2016;123:1421–1427. [DOI] [PubMed] [Google Scholar]
- 8.Price MO, Gupta P, Lass J, Price FW Jr. EK (DLEK, DSEK, DMEK): New frontier in cornea surgery. Ann Rev Vis Sci. 2017;3:69–90. [DOI] [PubMed] [Google Scholar]
- 9.Lass JH, Szczotka-Flynn LB, Ayala AR, et al. Cornea Preservation Time Study: methods and potential impact on the cornea donor pool in the United States. Cornea. 2015;34:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenwasser GO, Szczotka-Flynn LB, Ayala AR, et al. Effect of Cornea Preservation Time on Success of Descemet stripping automated endothelial keratoplasty: A randomized clinical trial. JAMA Ophthalmol. 2017;135:1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lass JH, Benetz BA, Verdier DD, et al. Corneal endothelial cell loss 3 years after successful Descemet stripping automated endothelial keratoplasty in the Cornea Preservation Time Study: A randomized clinical trial. JAMA Ophthalmol. 2017;135:1394–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terry MA, Aldave AJ, Szczotka LB, et al. Donor, recipient, and operative factors asssociated with graft success in the Cornea Preservation Time Study. Ophthalmology. 2018;125:1700–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pham C, Hellier E, Vo M, et al. Donor endothelial image quality in Optisol GS and Life 4°C. Int J Eye Banking. 2013;1:1–8. [Google Scholar]
- 14.Tran KD, Clover J, Ansin A, et al. Rapid warming of donor corneas is safe and improves specular image quality. Cornea. 2017;36:581–587. [DOI] [PubMed] [Google Scholar]
- 15.Benetz BA, Gal RL, Ruedy KJ, et al. Specular Microscopy Ancillary Study methods for donor endothelial cell density determination of Cornea Donor Study images. Curr Eye Res. 2006;31:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sayegh RR, Benetz BA, Lass JH. Specular microscopy In: Mannis MJ, Holland EJ, eds. Cornea: Fundamentals, Diagnosis, Management. Vol 1: Elsevier; 2016:160–179. [Google Scholar]
- 17.Nishimura JK, Hodge DO, Bourne WM. Initial endothelial cell density and chronic endothelial cell loss rate in corneal transplants with late endothelial failure. Ophthalmology. 1999;106:1962–1965. [DOI] [PubMed] [Google Scholar]
- 18.Patel SV. Graft survival and endothelial outcomes in the new era of endothelial keratoplasty. Exp Eye Res. 2012;95:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel SV. Keratoplasty for endothelial dysfunction. Ophthalmology. 2007;114:627–628. [DOI] [PubMed] [Google Scholar]
- 20.Lass JH, Benetz BA, Patel SV, et al. Donor, recipient, and operative factors influencing endothelial cell loss in the Cornea Preservation Time Study. JAMA Ophthalmol. doi: 10.1001/jamaophthalmol.2018.5669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wacker K, Baratz KH, Maguire LJ, et al. Descemet stripping endothelial keratoplasty for Fuchs’ endothelial corneal dystrophy: Five-year results of a prospective study. Ophthalmology. 2016;123:154–160. [DOI] [PubMed] [Google Scholar]
- 22.Patel SV, Hodge DO, Bourne WM. Corneal endothelium and postoperative outcomes 15 years after penetrating keratoplasty. Am J Ophthalmol. 2005;139:311–319. [DOI] [PubMed] [Google Scholar]
- 23.Patel SV, Diehl NN, Hodge DO, Bourne WM. Donor risk factors for graft failure in a 20-year study of penetrating keratoplasty. Arch Ophthalmol. 2010;128:418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price MO, Price FW, Jr. Endothelial cell loss after descemet stripping with endothelial keratoplasty influencing factors and 2-year trend. Ophthalmology. 2008;115(5):857–865. [DOI] [PubMed] [Google Scholar]
- 25.Brockmann T, Pilger D, Brockmann C, et al. Predictive factors for clinical outcomes after primary Descemet’s membrane endothelial keratoplasty for Fuchs’ endothelial dystrophy. Curr Eye Res. doi: 10.1080/02713683.2018.1538459) [DOI] [PubMed] [Google Scholar]
- 26.Coster DJ, Lowe MT, Keane MC, Williams KA. A comparison of lamellar and penetrating keratoplasty outcomes: a registry study. Ophthalmology. 2014;121:979–987. [DOI] [PubMed] [Google Scholar]