Abstract
For equal chromosome segregation, a pair of kinetochores on each duplicated chromosome must attach to microtubules connecting to opposite poles. The protein kinase Aurora B plays a critical role in destabilizing microtubules attached in a wrong orientation through phosphorylating kinetochore proteins. The mechanism behind this selective destabilization of aberrant attachments remains elusive. While Aurora B is most enriched on the centromere from prophase to metaphase, emerging evidence suggests the importance of Aurora B on microtubules in this process. Here I discuss two hypothetical models that explain the requirement of Aurora B on microtubules for selective destabilization of aberrant attachments; microtubule-induced substrate masking and treadmill-removal of Aurora B on microtubules proximal to polymerizing ends.
Introduction
To segregate multiple chromosomes equally to dividing cells, a bipolar spindle guides directional chromosome movements. At the centromere of each chromosome, the kinetochore establishes a load-bearing attachment to microtubules. Each kinetochore must capture plus ends of microtubules that are connected to one spindle pole, while its sister kinetochore must attach to microtubules linked to the other spindle pole (Fig.1). This configuration (amphitelic attachment) ensures equal distribution of sister chromatids upon their separation in anaphase. If both sister kinetochores attach to microtubules from the same pole (syntelic attachment), or one kinetochore attaches to microtubules from multiple poles (merotelic attachment), these aberrant attachments must be corrected before anaphase to prevent aneuploidy and formation of micronuclei. One of the key regulators of this error correction mechanism is the protein kinase Aurora B, which is essential for destabilizing erroneous attachments. Since Aurora B is most enriched on the centromere when error correction occurs in mitosis, models have proposed that the tension-dependent change in kinetochore substrate accessibility by Aurora B at the centromere dictates the detachment probability [1–3]. Here I review accumulating lines of evidence supporting the critical role of microtubule-mediated Aurora B regulation, and speculate on its contribution to the selective destabilization of aberrant microtubule attachments.
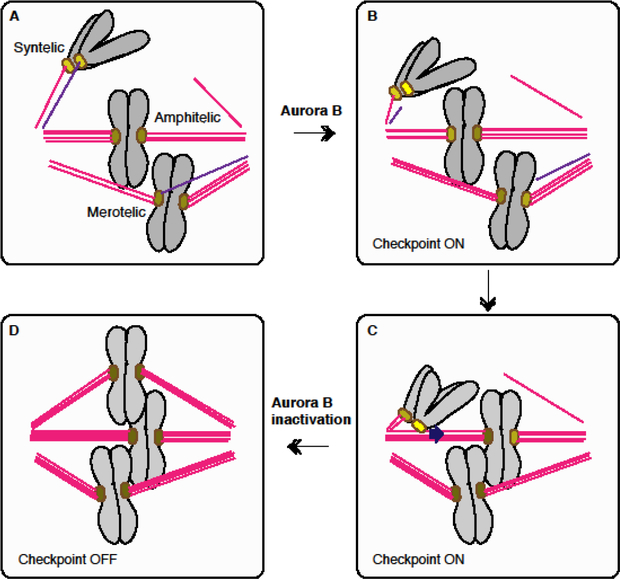
Figure 1. Aurora B-dependent error corrections of aberrant attachments.
A. Normal (amphitelic) and aberrant (syntelic and merotelic) attachments. B. Aurora B-mediated microtubule dissociation from aberrant attachments. Generation of an unattached kinetochore (yellow) activates the spindle checkpoint. C. Kinetochore attached to lateral side of microtubules can slide by the CENP-E motor toward the spindle equator [55]. Lateral attachment does not silence the checkpoint (yellow) [56*]. D. Conversion of lateral attachment to end on attachment, and stabilization of amphitelic attachments, which silence the spindle checkpoint. Color brightness of each kinetochore indicates the level of Aurora B-dependent phosphorylation (or checkpoint strength).
Basic mechanisms of correcting aberrant kinetochore-microtubule attachments
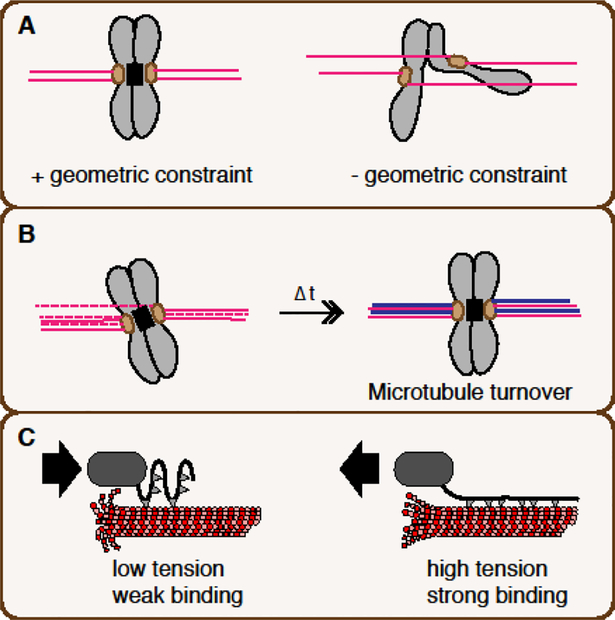
A mathematical model has indicated that geometric constraint of kinetochores and dynamic microtubule attachment can constitute a basic mechanism by which erroneous attachment is spontaneously corrected within finite time [4] (Fig. 2A, B). The geometric constraint, which limits attachment of microtubules preferentially to their proximal kinetochore, is sufficient to effectively accomplish bioriented attachment (Fig. 2A), while stochastic microtubule exchange can spontaneously displace aberrantly attached microtubules (Fig. 2B). However, this basic model may not readily explain kinetochore attachment control in meiosis I, where pairs of kinetochores on homologous chromosomes are not under apparent geometric constraint [5] (Fig. 2A). A prevailing model suggests that tension-dependent stabilization of kinetochore-microtubule attachment further contributes to the error correction mechanism [2,3].
Figure 2. Basic mechanistic principles behind selective stabilization of amphitelic attachments.
A. Geometric constraint. A pair of kinetochores linked through a geometric constraint (right) that interferes with attachment to microtubules coming from distal poles. The black box indicates the constraint, which may be promoted by the CPC at the centromere. In the absence of a geometric constraint (right), such as on paired homologous chromosomes during meiosis I, attachment from distal poles is enabled, resulting in merotelic attachment (and also syntelic attachment). B. Spontaneous microtubule turnovers. Each microtubule attachment has limited lifetime, and is spontaneously replaced by new attachment. A few aberrant attachments can be spontaneously replaced by correct attachment as the geometric constraint interferes with aberrant attachment. The turnover can be enhanced by Aurora B. C. Tension-induced enhancement of kinetochore-microtubule attachment (catch-bond mechanism). At a kinetochore under reduced tension (left), intrinsic microtubule binding is weak. At a kinetochore under tension (right), intrinsic microtubule binding is enhanced. Aurora B can weaken the microtubule-binding strength.
The tension model was initially substantiated by microneedle manipulation of chromosomes in grasshopper spermatocytes, showing that tension can stabilize kinetochore microtubule attachment [6]. Biophysical experiments with purified budding yeast kinetochores further demonstrated that kinetochores attached to depolymerizing ends are much more vulnerable to detachment than those attached to polymerizing ends are, but tension can stabilize kinetochore microtubule attachments to depolymerizing ends without ATP [7–9]. Therefore, the kinetochore has an intrinsic catch bond-like capacity to change its microtubule binding property depending on tension (Fig. 2C). However, in vivo, tension-dependent error correction depends on additional enzymatic reactions, whose major player is the protein kinase Aurora B.
Microtubule detachment by Aurora B-dependent phosphorylation of kinetochore substrates
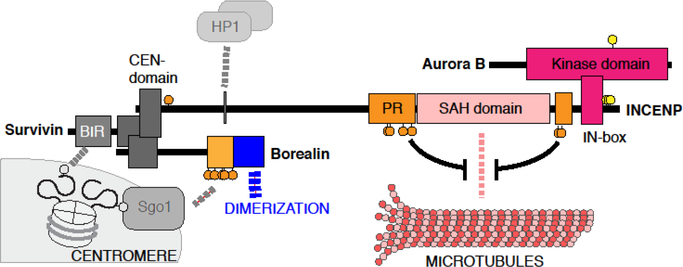
Aurora B, which forms the Chromosomal Passenger Complex (CPC) with INCENP, Borealin and Survivin (Fig.3), regulates a number of mitotic processes [3,10]. Upon mitotic entry, the CPC becomes enriched on the centromere between two pairs of sister kinetochores. Centromeric enrichment of the CPC depends on two kinds of histone phosphorylation marks. Survivin directly binds to histone H3 phosphorylated at Thr3 (H3T3ph), while Borealin binds to Sgo1, which is recruited to H2A phosphorylated at Thr120. At the metaphase-to-anaphase transition, the CPC dissociates from the centromere and relocalizes to the spindle midzone.
Figure 3. Members of the CPC; Aurora B, INCENP, Survivin and Borealin.
Aurora B interacts with INCENP at the IN-box domain, which allosterically activates Aurora B. Aurora B-dependent phosphorylation (yellow circles) at the Aurora B activation loop and on INCENP promotes full activation. The N-terminal CEN domain of INCENP interacts with Survivin and Borealin. This trimeric module targets the CPC to centromere-enriched histone phosphorylation marks; H3 phosphorylated at Thr3 recognized by the BIR domain of Survivin, and H2A phosphorylated at Thr120, which recruits Borealin through Sgo1 in a manner dependent on Cdk1-mediated phosphorylation at Borealin (orange circles). The SAH domain interacts with microtubules, while the PRD domain also supports microtubule binding, which is suppressed by Cdk1-dependent phosphorylation (orange circles). INCENP also interacts with HP1, which appears to facilitate substrate recognition.
Aurora B dependent phosphorylation of the Ndc80 (Hec1 in human) subunit of the Ndc80 complex, which is critical for supporting the load-bearing microtubule attachment, facilitates microtubule detachment [9,11,12]. In human cells, the N-terminal tail of Hec1, which contributes to microtubule binding together with the adjacent calponin homology domain, is phosphorylated at nine residues by Aurora B, and microtubule binding affinity gradually decreases as the number of phosphorylation sites increases [11]. In budding yeast, multisite phosphorylation of the Dam1 complex also plays an important role in microtubule detachment [9]. The phosphorylation status of Ndc80 and several other kinetochore proteins is sensitive to microtubule-mediated tension applied to the kinetochore [13–15]. A Förster Resonance Energy Transfer (FRET) based intramolecular tension-sensor established that the force applied at the kinetochore is generated by microtubule dynamics [16]. An intermolecular FRET based-measurement of Hec1-microtubule binding at the kinetochore in human cells visualized tension-induced microtubule binding, which is sensitive to Aurora B-dependent phosphorylation of Hec1 [17**].
The detachment probability is controlled by tension and Aurora B-dependent phosphorylation of kinetochore substrates. However, tension and complete dephosphorylation are not necessarily required for microtubule attachment. Even when tension is reduced by taxol, which stabilizes microtubule end dynamics, the majority of kinetochores maintain microtubule attachment, while Aurora B continuously generates a small number of unattached kinetochores, which activate the spindle checkpoint [18]. In cells expressing Hec1 with mutations mimicking a hyper-phosphorylated form (Hec1–9D), kinetochores can maintain attachment to depolymerizing ends [19*], although kinetochore microtubules become unstable and activate the checkpoint [20]. While Hec1 generally becomes dephosphorylated on metaphase kinetochores [13], phosphorylation of Hec1 at Ser69 is maintained and is important for suppressing formation of lagging chromosomes in anaphase, likely through destabilizing merotelic attachment [21*]. During prometaphase of mouse oocyte meiosis I, kinetochore phosphorylation levels remain high during prometaphase even on aligned chromosomes that are under full tension [22]. This kinetochore phosphorylation enables correction of merotelic attachments, which are frequently observed in female acentrosomal meiosis. Therefore, certain levels of kinetochore phosphorylation do not interfere with amphitelic attachment but rather help resolving merotelic attachment.
Once amphitelic attachment is accomplished, kinetochores must be dephosphorylated below a threshold to avoid stochastic microtubule detachment. Inhibiting Hec1 N-terminal phosphorylation (by Hec1–9A) stabilizes microtubule attachment regardless of the tension status and silences the spindle checkpoint even in the presence of unaligned chromosomes [23,24]. Therefore, in human cells, Hec1 dephosphorylation appears to be the key trigger for microtubule attachment stabilization, although the Dam1 dephosphorylation is more important in budding yeast [25]. This final microtubule stabilization, also helped by recruitment of the Ska complex (a plausible metazoan ortholog of the Dam 1 complex) and PP1 [26*,27*], is important for silencing the spindle checkpoint. The key question is how Aurora B-dependent phosphorylation is suppressed upon establishing amphitelic attachment.
Function of Aurora B on the centromere
Aurora B is activated by autophosphorylation of the activation loop and the C-terminal IN-box module of INCENP [28], and thus clustering of the CPC, such as by binding H3T3ph nucleosomes, can facilitate Aurora B activation [29–31]. Therefore, it has been speculated that the level of kinetochore phosphorylation can be regulated by the substrate accessibility of Aurora B activated on centromeric chromatin; as microtubules pull kinetochore substrates away from the centromeric Aurora B, phosphatases on the kinetochore win over the kinase to promote dephosphorylation [1–3].
However, recent observations question this model. First, the centromere-targeting module of the CPC is dispensable for efficient bipolar kinetochore attachment and viability in budding yeast [32]. Second, in human cells, it was shown that the critical function of the centromere-targeting module of the CPC is to maintain sister chromatid cohesion specifically at the centromere through recruiting Sgo1-PP2A [33**]. Interestingly, artificial targeting of INCENP to the kinetochore can result in chromosome misalignment with unstable kinetochore attachments, but this defect can be rescued by augmenting centromeric cohesion in the absence of the CPC on the centromere [33**]. The study suggests that the major function of the CPC at the centromere is to protect sister chromatid cohesion and provide physical connection between sister kinetochores. This ensures that the force acts on one kinetochore can be transmitted to its sister kinetochore, and/or that the geometrical constraint of kinetochores can be maintained. A similar role of the CPC at the centromere is reported in budding yeast, though this functionality is not essential for viability [32].
Regulation and importance of Aurora B on microtubules
Before anaphase, Cdk1 phosphorylates Thr59 of human INCENP, inhibiting interaction with a kinesin-like motor MKLP2, which targets the CPC to microtubules at the anaphase spindle midzone [34]. In addition, the single alpha helix (SAH) domain and its flanking phospho-regulatory (PR) domain of INCENP mediate microtubule association, while Cdk1-dependent phosphorylation of PR suppresses microtubule binding [35**–41**]. However, despite the CDK1-dependent suppression of INCENP-microtubule interaction, this interaction plays important roles even before anaphase. In Xenopus egg extracts, the microtubule-binding capacity of the SAH domain is essential for spindle assembly [36]. In HeLa cells, the SAH domain is needed for effective phosphorylation of Hec1, and for maintaining the spindle checkpoint in cells treated with taxol [35**]. Importantly, requirement for the SAH can be bypassed by replacement with the microtubule-binding domain of MAP4, but not with a dimerization module, which activates Aurora B without any localization [35**]. Furthermore, inhibiting Cdk1-dependent phosphorylation at the PR domain of INCENP interferes with checkpoint silencing [35**]. In budding yeast, an artificially generated minimal CPC, which is composed of Ipl1 (Aurora B) and a truncated, dimerized version of Sli15 (INCENP) just containing the SAH domain and the Aurora B-interaction domain, can support bioriented microtubule attachment [41**]. Other microtubule-binding proteins, such as the Ska complex, UBASH3B and EB1 also contribute to microtubule-dependent control of Aurora B activity during pre-anaphase [42–44].
Substrate masking and treadmill-removal models of suppressing Aurora B-dependent phosphorylation
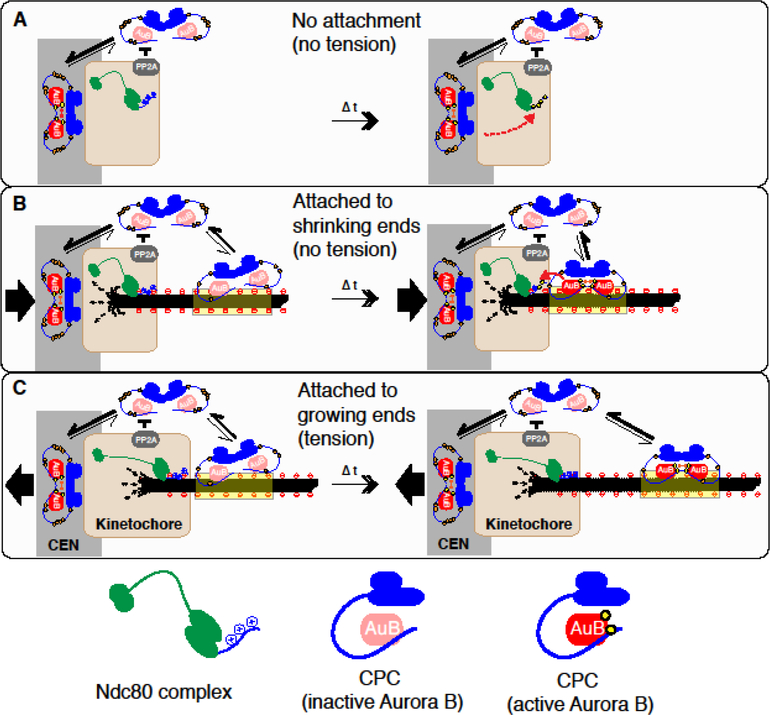
How can Aurora B on microtubules be a critical component of kinetochore phosphorylation and contribute to the tension-sensing mechanism? Here I postulate two models; substrate masking, and a treadmill-removal of Aurora B (Figure 4).
Figure 4. Models: substrate masking by microtubules and treadmill-removal of Aurora B.
A. Unattached kinetochore. Centromeric CPC enrichment activates Aurora B through autophosphorylation. Diffusion allows activated Aurora B (red) to access kinetochore substrates (orange dotted arrow), while its reaction is counteracted by PP2A at the kinetochore. The positively charged N-terminal tail of the Ndc80/Hec1 subunit of the Ndc80 complex (green) is recognized by Aurora B and microtubules. In the absence microtubules, Aurora B activated at the centromere can phosphorylate Ndc80.
B. Kinetochore attached to shrinking microtubule ends. The kinetochore is moving to a pole, and is under reduced tension. In the attached kinetochore, microtubules and Aurora B may compete to interact with the Ndc80 N-terminus, necessitating the microtubule-binding capacity of the CPC for effective Ndc80 phosphorylation (substrate masking by microtubules). The majority of the CPC with high Cdk1-dependent phosphorylation on INCENP (shown as orange circles) cannot bind to microtubules, but a small fraction of the CPC with reduced phosphorylation can bind to microtubules for a limited timeframe, during which Aurora B is activated. At the same duration, CPC with activated Aurora B comes closer to kinetochore substrates by treadmilling due to plus-end depolymerization, or stays close to the kinetochore (in the case of taxol-stabilized microtubules), facilitating accessibility of active Aurora B to its kinetochore substrates.
C. Kinetochore attached to growing microtubule ends. The kinetochore is moving away from a pole, and is under tension. The CPC with inactive Aurora B can be activated upon microtubule binding, but by the time when Aurora B is activated, it will already have been treadmilled away from the kinetochore, reducing its accessibility to kinetochore substrates (treadmill-removal). The CPC activated by microtubules or the centromeric chromatin may diffuse into the cytoplasm, but the high Cdk1 activity limits the rebinding to microtubules within the kinetochore.
Substrate masking.
On an unattached kinetochore (Fig.4A), Aurora B does not have to bind microtubules to phosphorylate the kinetochore substrates [13,14]. However, when kinetochores are attached to microtubules (Fig. 4B, C), I speculate that CPC-binding to microtubule becomes necessary. This may be due to the fact that key microtubule binding proteins, such as Ndc80, use positively charged residues to both interact with the acidic tubulin tails [45–47], and to be recognized by the basophilic kinase Aurora B [48]. Therefore, microtubule binding, which is stable enough to support load-bearing attachment, may mask Aurora B targeting positive charges. This process is corroborated by PP2A (and PP1) at the kinetochore, which counteracts Aurora B through dephosphorylating substrates [49]. As phosphatases recognize phosphorylated substrates, which consequentially dissociate from microtubules, microtubule binding would selectively affect recognition by Aurora B but not by phosphatases, shifting a balance toward dephosphorylation. Alternatively, microtubules may apply an indirect mechanism to mask substrates. For example, the Ndc80 complex can cluster upon binding to microtubules, and a segment of the N-terminal tail of Ndc80/Hec1 becomes occluded upon oligomerization [46]. Microtubule targeting of the CPC would facilitate substrate recognition to overcome these burdens [50].
Treadmill removal.
Aurora B can be activated by microtubules [29,36,39,51], but during prometaphase, high Cdk1 activity suppresses microtubule-binding of the CPC [34,35**]. However, a small fraction of the CPC can associate with microtubules where it can eventually become activated (Fig. 4B, C)[29,36,42,44]. At a microtubule segment proximal to a depolymerizing end, the density of the CPC with active Aurora B would be identical to that on an internal region (Fig. 3B). However, when Aurora B binds at a polymerizing end, by the time when it becomes activated, it would already have migrated to an internal position by treadmilling (Fig. 3C). If Aurora B-dependent INCENP phosphorylation also suppresses microtubule binding as shown in budding yeast [39,52], it would further restrict the diffusion-based accessibility of Aurora B that have been activated at distal locations. By this mechanism, activated Aurora B on microtubules will always be cleared from polymerizing ends.
On actively moving sister kinetochores that have established microtubule plus-ends, tension is observed at the trailing kinetochore attached to polymerizing microtubule ends, while tension is reduced on a leading kinetochore coupled to depolymerizing microtubules [53,54]. This may promote phosphorylation at the kinetochore attached to depolymerizing ends even after amphitelic attachment is achieved. However, on chromosomes aligned at the metaphase plate and undergoing oscillations, even kinetochores coupled to depolymerizing microtubules exhibit stronger Hec1-microtubule binding than those kinetochores whose tension is reduced by taxol or in monopolar spindles [17**]. As tension can selectively stabilize attachment to depolymerizing ends by an ATP-independent mechanism [7], the tension generated by bipolar attachment would be able to strengthen attachment to depolymerizing ends. Tension may also enhance the microtubule-mediated substrate masking effect, perhaps by increasing the microtubule-binding strength of the Aurora B targets. Once the balance shifts to dephosphorylation and stabilizes kinetochore attachment by recruiting the Ska complex and PP1, phosphorylation may not be able to re-occur [13].
The model may seem contradictory to a recent observation that suggests an importance of Aurora B at the centromere; inhibition of Haspin-dependent CPC recruitment at the centromere eliminates tension-dependent stabilization of Ndc80-microtubule binding, while it did not affect average binding affinity [17**]. However, as microtubule-dependent Aurora B activation facilitates centromeric localization of Aurora B [42–44], increasing the CPC pool at centromeres through microtubule-induced Aurora B activation may in turn facilitate phosphorylation of the kinetochore substrates, perhaps combined with a substrate-unmasking effect of basal phosphorylation via microtubule-bound Aurora B. Future studies including comparative measurement of Aurora B activation dynamics at microtubules and of the treadmilling rate, and biochemical analysis of substrate masking, should be able to validate these models.
Acknowledgement
H.F. thanks Sue Biggins, Pavan Choppakatla and Christian Zierhut for comments on this manuscript, and Dan Needleman and Tae Yeon Yoo for discussions. H.F. is supported by a grant from National Institutes of Health (R01GM075249, R01GM125302, GM121062). The content is solely the responsibility of the author and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Kelly AE, Funabiki H: Correcting aberrant kinetochore microtubule attachments: an Aurora B-centric view. Curr Opin Cell Biol 2009, 21:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lampson MA, Grishchuk EL: Mechanisms to Avoid and Correct Erroneous Kinetochore-Microtubule Attachments. Biology (Basel) 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krenn V, Musacchio A: The Aurora B Kinase in Chromosome Bi-Orientation and Spindle Checkpoint Signaling. Front Oncol 2015, 5:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaytsev AV, Grishchuk EL: Basic mechanism for biorientation of mitotic chromosomes is provided by the kinetochore geometry and indiscriminate turnover of kinetochore microtubules. Mol Biol Cell 2015, 26:3985–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitajima TS, Ohsugi M, Ellenberg J: Complete kinetochore tracking reveals error-prone homologous chromosome biorientation in mammalian oocytes. Cell 2011, 146:568–581. [DOI] [PubMed] [Google Scholar]
- 6.King JM, Nicklas RB: Tension on chromosomes increases the number of kinetochore microtubules but only within limits. Journal of Cell Science 2000, 113 Pt 21:3815–3823. [DOI] [PubMed] [Google Scholar]
- 7.Akiyoshi B, Sarangapani KK, Powers AF, Nelson CR, Reichow SL, Arellano-Santoyo H, Gonen T, Ranish JA, Asbury CL, Biggins S: Tension directly stabilizes reconstituted kinetochore-microtubule attachments. Nature 2010, 468:576–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller MP, Asbury CL, Biggins S: A TOG Protein Confers Tension Sensitivity to Kinetochore-Microtubule Attachments. Cell 2016, 165:1428–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarangapani KK, Akiyoshi B, Duggan NM, Biggins S, Asbury CL: Phosphoregulation promotes release of kinetochores from dynamic microtubules via multiple mechanisms. Proc Natl Acad Sci U S A 2013, 110:7282–7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carmena M, Wheelock M, Funabiki H, Earnshaw WC: The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nat Rev Mol Cell Biol 2012, 13:789–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaytsev AV, Sundin LJ, DeLuca KF, Grishchuk EL, DeLuca JG: Accurate phosphoregulation of kinetochore-microtubule affinity requires unconstrained molecular interactions. J Cell Biol 2014, 206:45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED: Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell 2006, 127:969–982. [DOI] [PubMed] [Google Scholar]
- 13.Deluca KF, Lens SM, Deluca JG: Temporal changes in Hec1 phosphorylation control kinetochore-microtubule attachment stability during mitosis. J Cell Sci 2011, 124:622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welburn JPI, Vleugel M, Liu D, Iii JRY, Lampson MA, Fukagawa T, Cheeseman IM: Aurora B Phosphorylates Spatially Distinct Targets to Differentially Regulate the Kinetochore-Microtubule Interface. Mol Cell 2010, 38:383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu D, Vader G, Vromans MJM, Lampson MA, Lens SMA: Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science 2009, 323:1350–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye AA, Cane S, Maresca TJ: Chromosome biorientation produces hundreds of piconewtons at a metazoan kinetochore. Nat Commun 2016, 7:13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoo TY, Choi JM, Conway W, Yu CH, Pappu RV, Needleman DJ: Measuring NDC80 binding reveals the molecular basis of tension-dependent kinetochore-microtubule attachments. Elife 2018, 7.** Through establishing a FRET-based measurement of Hec1-microtubule binding, the paper established a tension-dependent enhancement of Hec1-microtubule binding, which is reduced by Aurora B-dependent Hec1 phosphorylation. Amount of the CPC colocalized with Hec1 negatively correlates with microtubule-binding, and reducing centromeric Aurora B localization by inhibiting Haspin erased tension-dependent enhancement of microtubule binding.
- 18.Yang Z, Kenny AE, Brito DA, Rieder CL: Cells satisfy the mitotic checkpoint in Taxol, and do so faster in concentrations that stabilize syntelic attachments. J Cell Biol 2009, 186:675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long AF, Udy DB, Dumont S: Hec1 Tail Phosphorylation Differentially Regulates Mammalian Kinetochore Coupling to Polymerizing and Depolymerizing Microtubules. Curr Biol 2017, 27:1692–1699 e1693.* Using laser ablations, the paper monitors how kinetochores respond to a rapid change in kinetochore tension depending on Hec1 phosphorylation status. Phosphomimetic Hec1–9D makes a kinetochore more slippery when attached to polymerizing ends. Interestingly, Hec1–9D can track depolymerizing ends. The effect of Hec1 phosphorylation seems to differ between polymerizing ends and depolymerizing ends.
- 20.Sundin LJ, Guimaraes GJ, Deluca JG: The NDC80 complex proteins Nuf2 and Hec1 make distinct contributions to kinetochore-microtubule attachment in mitosis. Mol Biol Cell 2011, 22:759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeLuca KF, Meppelink A, Broad AJ, Mick JE, Peersen OB, Pektas S, Lens SMA, DeLuca JG: Aurora A kinase phosphorylates Hec1 to regulate metaphase kinetochore-microtubule dynamics. J Cell Biol 2018, 217:163–177.* Among nine Aurora B phosphorylation sites in Hec1 N-terminal tail, this paper shows that Ser69 is also phosphorylated by Aurora A. This is the only critical N-terminal Hec1 phosphorylation for supporting chromosome oscillation in metaphase, and inhibiting this phosphoryaltion interferes with chromosome segregation. Interestingly, a fraction of Aurora A is targeted to the kinetochore through binding to INCENP.
- 22.Yoshida S, Kaido M, Kitajima TS: Inherent Instability of Correct Kinetochore-Microtubule Attachments during Meiosis I in Oocytes. Dev Cell 2015, 33:589–602. [DOI] [PubMed] [Google Scholar]
- 23.Tauchman EC, Boehm FJ, DeLuca JG: Stable kinetochore-microtubule attachment is sufficient to silence the spindle assembly checkpoint in human cells. Nat Commun 2015, 6:10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Etemad B, Kuijt TE, Kops GJ: Kinetochore-microtubule attachment is sufficient to satisfy the human spindle assembly checkpoint. Nat Commun 2015, 6:8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin F, Wang Y: The signaling network that silences the spindle assembly checkpoint upon the establishment of chromosome bipolar attachment. Proc Natl Acad Sci U S A 2013, 110:21036–21041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janczyk PL, Skorupka KA, Tooley JG, Matson DR, Kestner CA, West T, Pornillos O, Stukenberg PT : Mechanism of Ska Recruitment by Ndc80 Complexes to Kinetochores. Dev Cell 2017, 41:438–449 e434.*The paper shows that N-terminal tail of Hec1 can be funcitonally divided in two segments; the very N-terminal segment is essential for chromosome alignment, while the C-terminal segment is required for recruiting the Ska complex to the kinetochore. The Ska complex appears to stabilize the oligomerized form of the Ndc80 complex, stabilizing microtubule attachment. Although the Ska complex recruitment to the Ndc80 complex is not required for chromosome alignment, it is required for checkpoint siliencing.
- 27.Cheerambathur DK, Prevo B, Hattersley N, Lewellyn L, Corbett KD, Oegema K, Desai A: Dephosphorylation of the Ndc80 Tail Stabilizes Kinetochore-Microtubule Attachments via the Ska Complex. Dev Cell 2017, 41:424–437 e424.*In C.elegans, Ndc80 N-terminal is dispensable for viability, but dephosphorylation is important to recruit the Ska complex, which is not required for amphitelic microtubule attachment, but is required for stabilizing microtubule attachment and damping chromosome oscillation. However, in C. elegans, this function is not required for checkpoint silencing.
- 28.Sessa F, Mapelli M, Ciferri C, Tarricone C, Areces LB, Schneider TR, Stukenberg PT, Musacchio A: Mechanism of Aurora B activation by INCENP and inhibition by hesperadin. Mol Cell 2005, 18:379–391. [DOI] [PubMed] [Google Scholar]
- 29.Kelly AE, Sampath SC, Maniar TA, Woo EM, Chait BT, Funabiki H: Chromosomal enrichment and activation of the aurora B pathway are coupled to spatially regulate spindle assembly. Dev Cell 2007, 12:31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly AE, Ghenoiu C, Xue JZ, Zierhut C, Kimura H, Funabiki H: Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science 2010, 330:235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zierhut C, Jenness C, Kimura H, Funabiki H: Nucleosomal regulation of chromatin composition and nuclear assembly revealed by histone depletion. Nat Struct Mol Biol 2014, 21:617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell CS, Desai A: Tension sensing by Aurora B kinase is independent of survivin-based centromere localization. Nature 2013, 497:118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hengeveld RCC, Vromans MJM, Vleugel M, Hadders MA, Lens SMA: Inner centromere localization of the CPC maintains centromere cohesion and allows mitotic checkpoint silencing. Nat Commun 2017, 8:15542.** Deleting the N-terminal centromere-targeting module of INCENP severely interfers with chromosome alignment. Artificial targeting of this mutant INCENP to the centromere by CENP-B does not rescue chromosome alignment with compromised centromere cohesion. Surprisingly, augmenting centromeric cohesion by Wapl depletion stabilizes kinetochore-microtubule attachment and chromosome alignment, but the checkpoint remains active. Thus, the centromeric CPC is important for maintining centromeric cohesion, while allowing checkpoint silencing by facilitating kinetochore dephosphorylation.
- 34.Hummer S, Mayer TU: Cdk1 negatively regulates midzone localization of the mitotic kinesin Mklp2 and the chromosomal passenger complex. Curr Biol 2009, 19:607–612. [DOI] [PubMed] [Google Scholar]
- 35.Wheelock MS, Wynne DJ, Tseng BS, Funabiki H: Dual recognition of chromatin and microtubules by INCENP is important for mitotic progression. J Cell Biol 2017, 216:925–941.** The paper shows that deletion of either N-terminal centromere targeting domain or the SAH domain inhibits the capacity of the CPC to phosphorylate kinetochore substrates and maintain the spindle checkpoint. The functionality of the SAH domain can be replaced by exogenous microtubule-binding domain of MAP4, while it is compromised by Cdk1-dependent phosphorylation of the PR domain.
- 36.Tseng BS, Tan L, Kapoor TM, Funabiki H: Dual detection of chromosomes and microtubules by the chromosomal passenger complex drives spindle assembly. Dev Cell 2010, 18:903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mackay AM, Eckley DM, Chue C, Earnshaw WC: Molecular analysis of the INCENPs (inner centromere proteins): separate domains are required for association with microtubules during interphase and with the central spindle during anaphase. J Cell Biol 1993, 123:373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Horst A, Vromans MJ, Bouwman K, van der Waal MS, Hadders MA, Lens SM: Inter-domain Cooperation in INCENP Promotes Aurora B Relocation from Centromeres to Microtubules. Cell Rep 2015, 12:380–387. [DOI] [PubMed] [Google Scholar]
- 39.Cormier A, Drubin DG, Barnes G: Phosphorylation regulates kinase and microtubule binding activities of the budding yeast chromosomal passenger complex in vitro. J Biol Chem 2013, 288:23203–23211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pereira G, Schiebel E: Separase regulates INCENP-Aurora B anaphase spindle function through Cdc14. Science 2003, 302:2120–2124. [DOI] [PubMed] [Google Scholar]
- 41.Fink S, Turnbull K, Desai A, Campbell CS: An engineered minimal chromosomal passenger complex reveals a role for INCENP/Sli15 spindle association in chromosome biorientation. J Cell Biol 2017, 216:911–923.** The paper demonstrates that the SAH domain and the IN-box module of INCENP/Sli15 are mimimum functional modules to support the essential function of Aurora B, and requirement of the centromere-targeting module and the PR module can be bypoassed by artificial dimerization. Requirement of the SAH domain suggests the essential role of INCENP-microtubule binding.
- 42.Krupina K, Kleiss C, Metzger T, Fournane S, Schmucker S, Hofmann K, Fischer B, Paul N, Porter IM, Raffelsberger W, et al. : Ubiquitin Receptor Protein UBASH3B Drives Aurora B Recruitment to Mitotic Microtubules. Dev Cell 2016, 36:63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Redli PM, Gasic I, Meraldi P, Nigg EA, Santamaria A: The Ska complex promotes Aurora B activity to ensure chromosome biorientation. J Cell Biol 2016, 215:77–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Banerjee B, Kestner CA, Stukenberg PT: EB1 enables spindle microtubules to regulate centromeric recruitment of Aurora B. J Cell Biol 2014, 204:947–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alushin GM, Ramey VH, Pasqualato S, Ball DA, Grigorieff N, Musacchio A, Nogales E: The Ndc80 kinetochore complex forms oligomeric arrays along microtubules. Nature 2010, 467:805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alushin GM, Musinipally V, Matson D, Tooley J, Stukenberg PT, Nogales E: Multimodal microtubule binding by the Ndc80 kinetochore complex. Nat Struct Mol Biol 2012, 19:1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ciferri C, Pasqualato S, Screpanti E, Varetti G, Santaguida S, Dos Reis G, Maiolica A, Polka J, De Luca JG, De Wulf P, et al. : Implications for kinetochoremicrotubule attachment from the structure of an engineered Ndc80 complex. Cell 2008, 133:427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alexander J, Lim D, Joughin BA, Hegemann B, Hutchins JR, Ehrenberger T, Ivins F, Sessa F, Hudecz O, Nigg EA, et al. : Spatial exclusivity combined with positive and negative selection of phosphorylation motifs is the basis for context-dependent mitotic signaling. Sci Signal 2011, 4:ra42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saurin AT: Kinase and Phosphatase Cross-Talk at the Kinetochore. Front Cell Dev Biol 2018, 6:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noujaim M, Bechstedt S, Wieczorek M, Brouhard GJ: Microtubules accelerate the kinase activity of Aurora-B by a reduction in dimensionality. PLoS One 2014, 9:e86786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fuller BG, Lampson MA, Foley EA, Rosasco-Nitcher S, Le KV, Tobelmann P, Brautigan DL, Stukenberg PT, Kapoor TM: Midzone activation of aurora B in anaphase produces an intracellular phosphorylation gradient. Nature 2008, 453:1132–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakajima Y, Cormier A, Tyers RG, Pigula A, Peng Y, Drubin DG, Barnes G: Ipl1/Aurora-dependent phosphorylation of Sli15/INCENP regulates CPC-spindle interaction to ensure proper microtubule dynamics. J Cell Biol 2011, 194:137–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dumont S, Salmon ED, Mitchison TJ: Deformations within moving kinetochores reveal different sites of active and passive force generation. Science 2012, 337:355–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki A, Badger BL, Haase J, Ohashi T, Erickson HP, Salmon ED, Bloom K: How the kinetochore couples microtubule force and centromere stretch to move chromosomes. Nat Cell Biol 2016, 18:382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kapoor TM, Lampson MA, Hergert P, Cameron L, Cimini D, Salmon ED, McEwen BF, Khodjakov A: Chromosomes can congress to the metaphase plate before biorientation. Science 2006, 311:388–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuhn J, Dumont S: Spindle assembly checkpoint satisfaction occurs via end-on but not lateral attachments under tension. J Cell Biol 2017, 216:1533–1542.* It has not been clear if kinetochore attachment to the lateral side of microtubules can silence the spindle checkpoint. Through monitoring kinetochore localization of the checkpoint proteing Mad1, a signature of checkpoint activation, together with microtubules by live microscopy in rat-kangaroo PtK2 cells, this study demonstrates that lateral attachment can exert tension at the kinetochore, but this cannot trigger Mad1 dissociation. End-on microtubule attachment triggers Mad1 dissoation.