Abstract
We aimed to analyze 10-year experience of WAIHA patients at a single referral center in Turkey. Clinical data, survival outcome of sixty patients who were diagnosed with WAIHA were retrospectively analyzed. All the patients were direct antiglobulin test (DAT) positive. In 21 (30%) patients, IgG plus C3d DAT positivity was documented. 16 patients were secondary WAIHA and most common underlying causes were lymphoproliferative diseases (5 patients) and connective tissue disease (8 patients). Corticosteroids were first choice as a first line therapy with 54.5% CR and 40.2% PR rates. 43.3% of the patients relapsed after a median 12 months. In relapsed patients, rituximab and splenectomy achieved 85% overall response rates. The median OS was not reached. The median DFS was 40 months (95% CI, 19.6–60.4). OS and DFS at 36 months were 89.6% and 51.1%, respectively. DFS at 36 months was lower in patients with IgG plus C3d positive DAT than patients with only positive Ig G DAT (36 vs. 54%) but this difference could not reach statistical significance (p = 0.23). WAIHA was a rare disease with a good prognosis. Corticosteroids were the first option and splenectomy and rituximab received good responses in relapsed patients. Attention should be paid especially in patients with IgG plus C3d DAT positivity since lower DFS were reported. Characteristics and pathogenesis of patients with IgG plus C3d DAT positivity was still an obscure.
Keywords: Warm autoimmune hemolytic anemia, Treatment, Mixed positive direct antiglobulin test
Introduction
Warm autoimmune hemolytic anemia (WAIHA) is an acquired disease, characterized by premature destruction of the red blood cells (RBCs) due to mainly IgG type autoantibodies [1, 2]. It is a rare disease with an incidence of 0.8 per 100.000 people per year [3]. It can be classified according to absence or presence of an underlying cause as primary or secondary, respectively. Secondary WAIHA, that accounts for half of the cases, can presente during the course of the underlying disease or it can be the first manifestation [1, 4, 5]. Therefore, a careful investigation is essential to rule out an underlying disease, which creates a great dilemma for the clinician.
The diagnosis is mainly based on the coexistence of anemia with positive direct antiglobulin test (DAT), reticulocytosis, high lactate dehydrogenase (LDH) and indirect bilirubin. Since WAIHA is a rare and heterogeneous disease, treatment recommendations mainly depend on a few prospective and retrospective studies [5–10]. Corticosteroids are the mainstay of first line treatment [6, 7] but steroid dependence and refractoriness still constitute a problem in 15–20% of the patients [11, 12]. In refractory patients, treatment options are diverse and decisions should be made on individual basis [6–8, 10].
The aim of this study is to document a 10-year experience of WAIHA patients at a single referral center in Turkey after the introduction of rituximab into the treatment.
Material and Method
Patients
Sixty patients who were diagnosed with WAIHA between January 2007 and January 2017 were enrolled into the study. The diagnosis was determined by anemia (hemoglobin [Hb] < 12 g/dl) associated with autoimmune hemolysis. The presence of autoimmune hemolysis was demonstrated by positive DAT, reticulocytosis, high LDH and indirect bilirubin levels and low haptoglobulin [13]. In patients with positive DAT (+ 3/+ 4 positivity), we routinely studied specific IgG and C3d DAT. The cut-off value for Ig G DAT positivity was accepted as + 3 positive result. Cohort was subdivided into two groups, patients with only IgG positive DAT and patients with IgG plus C3d positive DAT (mixed DAT). Exclusion criteria were negative DAT, drug-induced WAIHA, patients with only positive C3d DAT to rule out cold AIHA, and patients with Evans Syndrome [14].
Underlying causes were searched by rheumatologic markers including antinuclear, antiphospholipid, and anticardiolipin antibodies and extractable nuclear antigen (ENA) panel; and serologic tests for human immunodeffiency virus (HIV), hepatitis B virus (HBV) and hepatitis C virus (HCV). Imaging methods including ultrasonography, computed tomography, and lymph node or bone marrow biopsy were performed wherever necessary. All the data of the patients were retrospectively recorded from the archives.
Response Criteria
All the patients were evaluated on the first and third weeks of the therapy according to Gruppo Italiano Malattie e Matologiche dell’Adulto (Italian Group for Adult Hematologic Diseases- GIMEMA) (14). Complete response (CR) was defined as Hb ≥ 12 g/dl and normal hemolytic parameters including LDH, reticulocyte, haptoglobulin and indirect bilirubin. Partial response (PR) was defined as Hb ≥ 10 g/dl or at least 2 g/dl increase in Hb level from the onset of treatment. Relapse was considered when Hb level ≤ 10 g/dl or at least 2 g/dl decrease was documented. Overall survival (OS) was the time from diagnosis until death from any causes. Disease free survival (DFS) was defined as time from CR or PR to relapse.
Statistical Analysis
Statistical analysis was performed by SPSS Statistics 20. Chi square, Student’s t and Mann–Whitney U tests were used for comparisons between groups according to characteristics of the variables. Survival analysis was performed by log rank test and Kaplan–Meier curves. P values less than 0.05 were accepted as statistically significant.
Results
Patients’ Characteristics and Laboratory Tests at Diagnosis
Sixty patients (38 female, 22 male) diagnosed with WAIHA were included. The median age was 52 (range: 20–85) years old. The median Hb at diagnosis was 7 g/dl (range; 2.8–11.2 g/dl). The patients were divided into two groups according to Hb levels. Patients with Hb < 7 g/dl and ≥ 7 g/dl were classified as severe (25 patients) and non-severe WAIHA (35 patients), respectively. The type and the dose of the steroid does not differ between the severe and non-severe groups. We preferred intravenous route in the severe forms with the same dose 1 mg/kg/day. In symptomatic patients with severe anemia erythrocyte transfusion was added to the standard steroid therapy. When we compared the two groups, we could not document a statistically significant difference in terms of sex, age, and presence of secondary diseases. The number of patients who were transfused was similar in both groups (13 (46.4%) patients in severe group and 15 (53.6) in non-severe group; p > 0.05).
The median reticulocyte level was determined as 9% (1.1–40). Eight of the patients were presented with reticulocytopenia, and five of them were presented with severe anemia.
All the patients were IgG DAT positive. Mixed positive DAT, defined as IgG plus C3d positive DAT, was reported in 21 (30%). Among patients with mixed positive DAT, ten patients (50%) were presented with severe anemia. The patients’ characteristics and laboratory tests at the time of diagnosis was summarized in Table 1.
Table 1.
Characteristics of the patients and comparison of primary and secondary WAIHA cases
| Characteristics of the patients | All patients | Secondary WAIHA | Primary WAIHA | p value |
|---|---|---|---|---|
| Sex (n) | ||||
| Female/male | 38/22 | 9/7 | 29/15 | 0.492 |
| Age (median, range) (years old) | 52 (20–85) | 55 (20–76) | 51 (30–85) | 0.544 |
| Hb (gr/dl) (median, range) | 7 (2.8–11.2) | 6.95 (3.5–11.2) | 7.1 (2.8–10.1) | 0.698 |
| Thrombocyte (× 109/L) median, range | 459 (158–758) | 231 (166–466) | 262 (158–758) | 0.186 |
| Reticulocyte (%) median, range | 9 (1.1–40) | 9 (0.7–33) | 10 (0.7–40) | 0.629 |
| LDH (IU/L) | 483 (280–1896) | 310 (280–911) | 546 (345–1896) | 0.205 |
| Indirect bilirubin (mg/dl) | 3.3 (2.4–6.2) | 3.2 (2.4–5.5) | 3.3 (2.8–6.2) | 0.451 |
| Mixed positive DAT test (n (%30) | 21 (30%) | 11 (68.7%) | 10 (22.7%) | 0.04 |
| Median number of erythrocyte suspension units transfused | 3 (1–6) | 2(1–4) | 2(1–6) | 0.31 |
WAIHA warm autoimmune hemolytic anemia, LDH lactate dehydrogenase, DAT direct antiglobulin test, Hb hemoglobin and RBC red blood cell
Underlying Causes and Comparison of Primary and Secondary WAIHA Patients (Table 1)
Among 60 patients, underlying causes were documented in 16 patients (26%) (Table 2). The primary and secondary groups did not differ statistically with regard to sex, Hb level, and biochemical parameters.
Table 2.
The underlying causes and relation to the initiation of WAIHA
| Underlying causes | Number of patients |
|---|---|
| SLE | |
| Presented with WAIHA | 3 |
| WAIHA during the course of SLE | 2 |
| CLL | |
| Presented with WAIHA | 2 |
| WAIHA during the course of CLL | 3 |
| Sjogren syndrome | |
| Presented with WAIHA | 1 |
| WAIHA during the course of SJogren syndrome | 1 |
| Psoriatic arthritis | |
| Presented with WAIHA | 1 |
| Hepatitis C | |
| Presented with WAIHA | 1 |
| Cirrhosis | |
| Presented with WAIHA | 2 |
In 7 of 16 patients with underlying disease (43.7%), WAIHA was the first finding and associated diseases were diagnosed during the work up of WAIHA (Table 2). 68.7% of the secondary cases and 22.7% of the primary cases positive for mixed DAT (p = 0.04) (Table 1). All the patients with connective tissue disease were female. During the long term follow-up of the patients, one patient with severe WAIHA was diagnosed with diffuse large B cell lymphoma (DLBCL) 5 years after diagnosis of WAIHA while she was in remission in terms of WAIHA (not included in secondary patients).
Treatment and Response
All patients except three were treated with prednisolone as a first line therapy. Among these three patients, two of them with a diagnosis of SLL were treated with the R-CVP (rituximab, cyclophosphamide, prednisolone) regimen. One patient with compensated anemia (Hb: 11.4 g/dl) was followed up without treatment. No exacerbation was documented during a follow-up of 38 months.
The patients were treated with prednisolone 1 mg/kg/day. The median duration of steroid treatment was 3 months (range: 2–96). The patient treated with steroid for 96 months had SLE and 4 mg; an every-other-day schedule was preferred to control the symptoms of SLE. The overall response rate was 94.7%. CR and PR rates were 54.5% and 40.2%, in the total cohort, respectively. The CR rates at primary and secondary group were 56.8% and 50% respectively which was not statistically different (p = 0.41). The CR and PR rates at patients with mixed positive DAT test was 47% and 47% respectively. Although CR rates at mixed positive DAT test was lower (47% vs. 56% at the patients with only Ig G positivity), this difference could not reach statistical significance. The increase in Hb level was median 4 (range: 2–10)g/dl at the first follow-up visit. The increase in Hb level was higher in patients with severe WAIHA than patients with the non-severe form (median 6 g/dl vs. 4 g/dl, p < 0.05).
During the follow-up period, 43.3% of the patients were relapsed after a median of 12 months (range: 3–24). In this group, 12 of the patients had an response to steroid for more than 12 months at the first attack. The median number of relapses per patient was 3 (range: 2–12). Median number of attacks did not differ between primary and secondary cases or in patients with severe or non-severe forms. In nine of the relapsed patients, another course of steroids was given. Seven patients achieved CR. Two patients were refractory and treated with rituximab and splenectomy.
Options in Relapses
Splenectomy was performed in 7 patients. The median time to splenectomy was 13 months (range: 6–28 months). The total response rate was 85.7% (42.1% CR and 43.6 PR) after splenectomy.
Rituximab was given as 375 mg/m2/day in four doses once a week in seven patients. Rituximab achieved an overall response rate of 85% (42.5% CR and 42.5% PR). Two of the patients relapsed after rituximab therapy, and CR was achieved in these patients in another cycle. One patient who was refractory to rituximab was treated with azathioprine; one patient who relapsed after rituximab therapy was successfully treated with splenectomy, and CR was documented.
Immunosuppressive therapy with azathioprine was needed in three of the patients, and only compensated hemolysis could be achieved in these highly refractory patients. We could not document any risk factors for poor response or relapse.
Survival Analysis
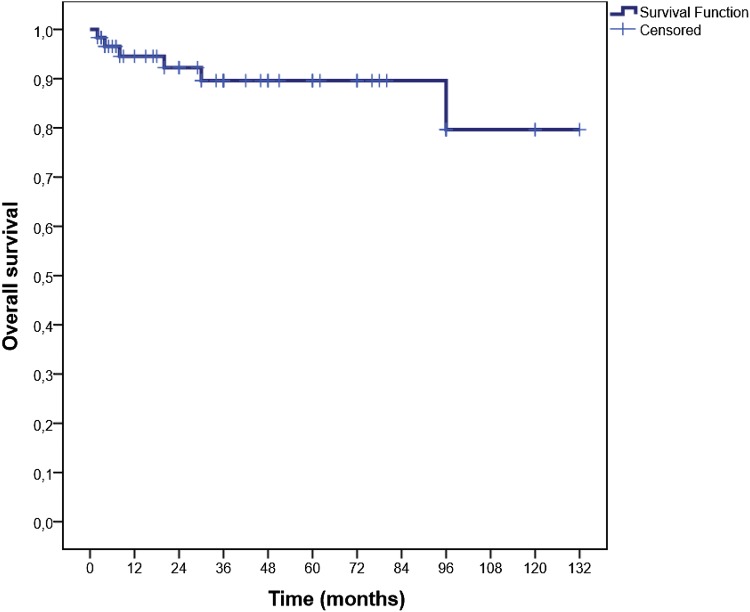
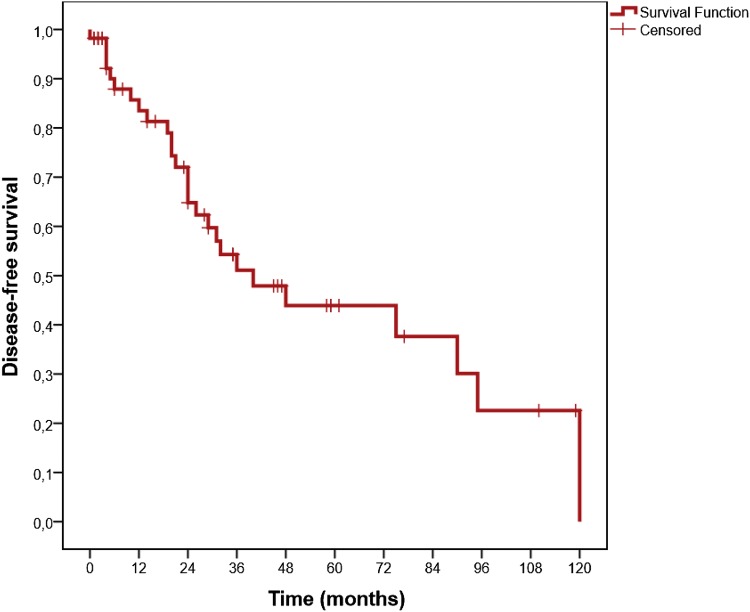
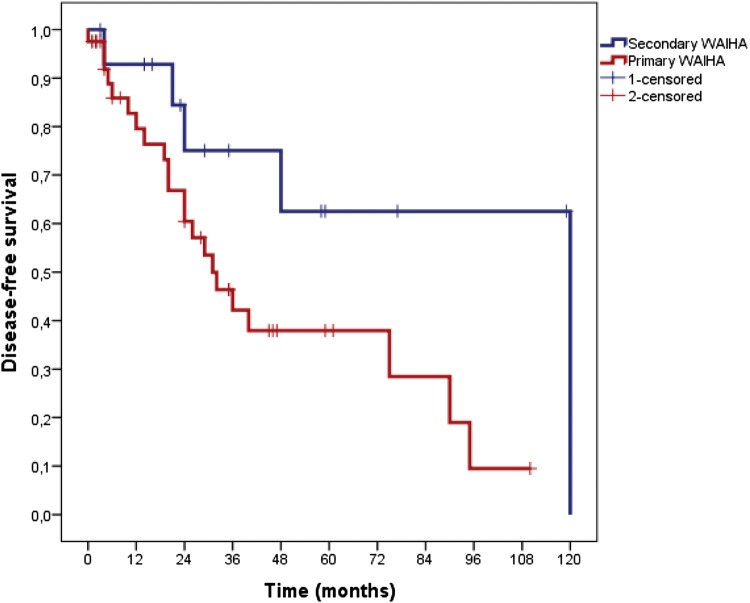
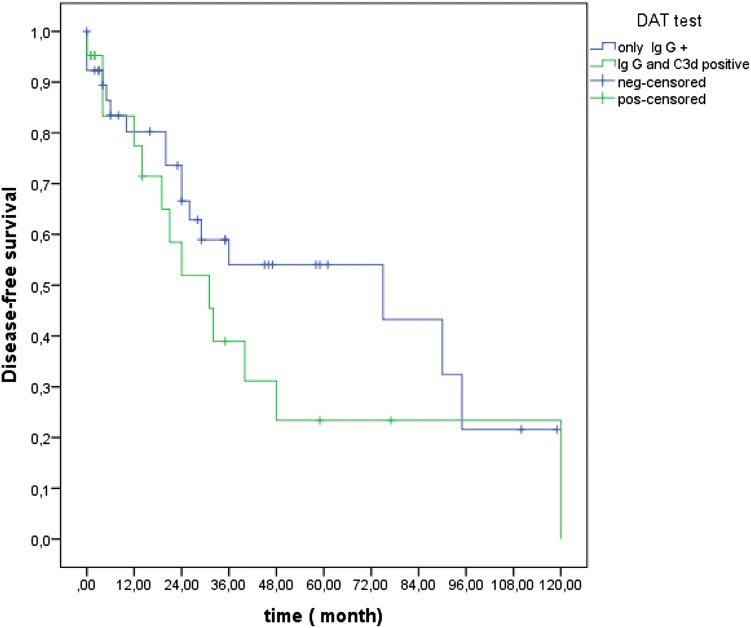
The median time of follow-up was 36 months (range: 12–132). The median OS was not reached. The median DFS was 40 months (95% CI; 19.6–60.4). OS and DFS at 36 months was 89.6% (Fig. 1) and 51.1% (Fig. 2), respectively. In patients with severe and non-severe forms, the DFS at 36 months did not differ statistically (52.1% in severe and 47.9% in non-severe group; p = 0.55). DFS at 36 months was higher in secondary (75%) group than in primary (42.2%) patients (p = 0.04; Fig. 3). DFS at 36 months was lower in patients with mixed positive DAT than patients with only positive IgG DAT (36% vs. 54%), but this difference could not reach statistical significance (p = 0.23; Fig. 4).
Fig. 1.
Kaplan Meiyer curves for overall survival in all patients
Fig. 2.
Kaplan Meiyer curves for disease free survival in all patients
Fig. 3.
Kaplan Meiyer curves of disease- free survival in secondary and primary WAIHA patients
Fig. 4.
Kaplan Meiyer curves of disease- free survival in patients with only Ig G DAT + and mixed positive DAT
During the follow-up period, four patients died. One patient with splenectomy and steroid dependence died due to pneumonia and sepsis. One patient with hepatic cirrhosis died because of worsening of the primary disease. The hemolysis was compensated at the time of death. We could not provide the cause of death in two patients, but they were at PR at the last visit.
Discussion
Although WAIHA is a benign disease, it can be a medical challenge due to the rarity of the disease, diagnostic difficulties, and heterogeneity of treatment options in especially refractory cases. Most of the studies in the literature consist of heterogeneous groups of patients [4, 5, 12, 14]. Under these circumstances, we aimed to determine characteristic and treatment results in relatively a homogeneous group of Turkish WAIHA patients.
We analyzed 60 WAIHA patients. The median age was 52, and female patients constitute 63% of the cohort. In the literature, the median age at the diagnosis ranges between 30 and 53 years of age [5, 11, 12]. This wide range is attributed to the heterogeneity of the cohorts included in different studies. The female predominance was also remarkable in other studies [1, 14, 15]. This predominance may be attributed to the affinity of females to all autoimmune diseases.
Laboratory results regarding hemolytic parameters were compatible with the literature [5, 16]. Although reticulocytosis is the hallmark of AIHA, we documented reticulocytopenia in eight (13.3%) patients. In the literature, reticulocytopenia was reported in 20–25% [12, 14, 16] of the patients. Although the exact pathogenesis could not be explained, it is possibly due to apoptosis or autoimmune destruction of RBC precursors in bone marrow or hemolysis of the reticulocytes in the peripheral blood. Due to ineffective compensation of hemolysis, it is accepted as clinical emergency [14, 17].
Limited data was available in WAIHA patients with mixed DAT positivity [11, 14, 16]. We found out that 30% of the patients were presented with mixed positive DAT. In our study, severe anemia and secondary forms were more frequent in these patients. In the literature, mixed positive DAT was reported in 25–50% of the patients. In the largest study consisted of 308 AIHA patients, the Hb levels were statistically lower (median 6.9 g/dl) in the mixed positive DAT group [14]. Similar to our results, two studies also documented a higher incidence of mixed DAT positivity in secondary forms [11, 16]. Although the pathogenesis was not well documented, the existence of another autoimmune disease may be a trigger for activating a complementary system [16, 18].
Underlying causes were detected in 26% of the patients, and the most common secondary cause was connective tissue disease. In the literature, secondary AIHA accounts for 21–77% of all cases [5, 11, 12, 16]. This wide range is mostly due to heterogeneity of the patients and laboratory tests performed to determine secondary cases at the initial diagnosis. The most common underlying causes were connective tissue [5, 16] and lymphoproliferative diseases [11]. In ten patients in our cohort, the underlying disease was diagnosed during the initial workup of WAIHA, which drew attention to the importance of a diagnostic workup of AIHA patients. In series with a detailed workup including CT scans and sophisticated tests to rule out lymphoproliferative diseases, the incidence of secondary cases increased from 51 to 62% [11]. Another important point in WAIHA patients is that underlying causes may be evident during the follow-up period [16]. In a study from Korea, conversion of primary cases to secondary ones was reported in 56% of the patients. In our study, only one patient was diagnosed as DLBCL after 5 years after the first attack. At the time of DLBCL diagnosis, hemolysis was in remission. So there is a contradiction in this case, if it is a coincidental finding.
Concerning the treatment, phase 3 prospective trials are sparse in the literature. Recommendations for treatment were based on few prospective and mostly retrospective analyses. Steroids were first option. We report an overall response of 94.7%. CR and PR were 54.5% and 40.2%, respectively. Response rates did not correlate with age, sex, Hb level during diagnosis, nor with the presence of underlying cause as reported in the literature [12]. Rattarittamrong et al. analyzed the characteristics and long-term outcomes of WAIHA patients in Thailand. They reported an overall response rate as 96%, and no difference was documented between primary and secondary cases [1]. Roumier et al. [11] confirmed similar response rates. Steroids achieved 50% CR and 38% PR, which was compatible with our results. In the subgroup analysis of the GIMEMA study by Barcellini, an overall response rate of 80% was reported in WAIHA patients. The response rate was lower in WAIHA patients with mixed positive DAT similar to our results [14]. We treated patients with steroids for a median of 3 months. Although many physicians tend to discontinue steroids earlier due to adverse effects, a long-term treatment duration is associated with lower relapse rates [14, 19]. So, longer steroid therapy and slower tapering should be considered in appropriate settings.
Although steroid responsiveness is high in WAIHA patients, relapses constitute a big dilemma in these patients [1, 14, 19]. Relapses occurred in about half of the patients [1]. We demonstrated a relapse in 43.3% of the patients. A number of attacks did not differ statistically in our cohort between primary and secondary forms and also in severe and non-severe groups.
Besides another episode of steroid administration, splenectomy, rituximab and immunosuppressive agents are options in relapsed patients [20, 21]. We treated sixteen patients with second line treatment (seven patients with rituximab, six patients with splenectomy, and three patients with azathioprine). Rituximab and splenectomy achieved 85% response rates in our study that was compatible with the literature [11, 14]. Relapses may also be observed after rituximab therapy, and another cycle may be administered with good response rates [22] as similar in our study.
Concerning immunosuppressive agents, only 5% of the patients were treated with azathioprine. Only compensated hemolysis was achieved in this group. Response rates were low with immunosuppressive treatments, and relapses were much more common [11].
Four patients (6.6%) died during the follow-up. One of the splenectomized patients died because of pneumonia and sepsis. Risk of infections were also reported to be higher in splenectomized patients, especially those who were on steroid therapy, and sepsis was one of the most important causes of death [1, 11, 14].
Data regarding OS and DFS rates was sparse. We demonstrated OS at 36 months as 89.6% and similar results reported by Rattarittamrong et al. [1]. The median DFS was 40 months (95% CI; 19.6–60.4); DFS at 36 months was 51.1%. Although DFS did not differ between severe and non-severe forms, DFS at 36 months was lower in primary WAIHA patients. Contrary to our findings, Alonso et al. [16]. reported higher median DFS rates for primary WAIHA patients. Beside this study, lower DFS levels in secondary cases could not be confirmed by other studies [1].
Our results also revealed that patients with mixed positive DAT had lower DFS than patients with only IgG DAT positivity. The adverse effect of mixed positive DAT was also reported in the GIMEMA study [14]. This effect could be explained by stronger stimulation of the autoimmune system [16].
Our study also had some limitations. First, it was a retrospective study: so, we could not reach all the data of the patients, and losing the follow-up was another problem. Second, we could not include patients with a negative DAT to rule out non-immune reasons for hemolysis. Beside these limitations, our study had relatively homogeneous group, consisting of only WAIHA patients, and a relatively large cohort, considering the rarity of the disease.
In conclusion, WAIHA is a rare disease with a heterogeneous presentation. Underlying causes should be ruled out with detailed history-taking and essential laboratory tests, because WAIHA may be the first sign of an underlying disease as we documented in ten patients. Although 94.7% response rate was achieved with steroids, relapses constituted a problem in 43.3% of the patients. In relapsed patients, rituximab and splenectomy are good options with a more than 80% response rate. Attention should be especially paid with patients with mixed DAT positivity, since a lower DFS was reported in literature. Characteristics and pathogenesis of patients with mixed DAT positivity is still an obscure.
Compliance with Ethical Standards
Conflict of interest
Authors Fergün Yılmaz, Demet Kiper, Meltem Koç, Tuğçe Karslı, Merve Kılınç, Fusun Gediz, Tayfur Toptaş, Bahriye Payzın declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rattarittamrong E, Eiamprapai P, Tantiworawit A, Rattanathammethee T, Hantrakool S, Chai-Adisaksopha C, Norasetthada L. Clinical characteristics and long-term outcomes of warm-type autoimmune hemolytic anemia. Hematology. 2016;21(6):368–374. doi: 10.1080/10245332.2016.1138621. [DOI] [PubMed] [Google Scholar]
- 2.Kamesaki T. Molecular mechanisms of autoimmune hemolytic anemia. RinshoKetsueki. 2015;56(7):846–854. doi: 10.11406/rinketsu.56.846. [DOI] [PubMed] [Google Scholar]
- 3.Klein NP, Ray P, Carpenter D, Hansen J, Lewis E, Fireman B, et al. Rates of autoimmune diseases in Kaiser Permanente for use in vaccine adverse event safety studies. Vaccine. 2010;28(4):1062–1068. doi: 10.1016/j.vaccine.2009.10.115. [DOI] [PubMed] [Google Scholar]
- 4.Genty I, Michel M, Hermine O, Schaeffer A, Godeau B, Rochant H. Characteristics of autoimmune hemolytic anemia in adults: retrospective analysis of 83 cases. Rev Med Interne. 2002;23(11):901–909. doi: 10.1016/S0248-8663(02)00688-4. [DOI] [PubMed] [Google Scholar]
- 5.Baek SW, Lee MW, Ryu HW, Lee KS, Song IC, Lee HJ, Yun HJ, Kim S, Jo DY. Clinical features and outcomes of autoimmune hemolytic anemia: a retrospective analysis of 32 cases. Korean J Hematol. 2011;46(2):111–117. doi: 10.5045/kjh.2011.46.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lechner K, Jäger U. How I treat autoimmune hemolytic anemias in adults. Blood. 2010;116(11):1831–1838. doi: 10.1182/blood-2010-03-259325. [DOI] [PubMed] [Google Scholar]
- 7.Crowther M, Chan YL, Garbett IK, Lim W, Vickers MA, Crowther MA. Evidence-based focused review of the treatment of idiopathic warm immune hemolytic anemia in adults. Blood. 2011;118(15):4036–4040. doi: 10.1182/blood-2011-05-347708. [DOI] [PubMed] [Google Scholar]
- 8.Maung SW, Leahy M, O’Leary HM, Khan I, Cahill MR, Gilligan O, Murphy P, McPherson S, Jackson F, Ryan M, Hennessy B, McHugh J, Goodyer M, Bacon L, O’Gorman P, Nee A, O’Dwyer M, Enright H, Saunders J, Saunders J, O’Keeffe D. A multi-centre retrospective study of rituximab use in the treatment of relapsed or resistant warm autoimmune haemolytic anaemia. Br J Haematol. 2013;163(1):118–122. doi: 10.1111/bjh.12486. [DOI] [PubMed] [Google Scholar]
- 9.Birgens H, Frederiksen H, Hasselbalch HC. A phase III randomized trial comparing glucocorticoid monotherapy versus glucocorticoid and rituximab in patients with autoimmune haemolytic anaemia. Br J Haematol. 2013;163(3):393–399. doi: 10.1111/bjh.12541. [DOI] [PubMed] [Google Scholar]
- 10.Michel M, Terriou L, Roudot-Thoraval F, et al. A randomized and double blind controlled trial evaluating the safety and efficacy of rituximab fo rwarm autoimmune hemolytic anemia in adults (the RAIHA study) Am J Hematol. 2017;92(1):23–27. doi: 10.1002/ajh.24570. [DOI] [PubMed] [Google Scholar]
- 11.Roumier M, Loustau V, Guillaud C, Languille L, Mahevas M, Khellaf M, Limal N, Noizat-Pirenne F, Godeau B, Michel M. Characteristics and outcome of warm autoimmune hemolytic anemia in adults: new insights based on a single-center experience with 60 patients. Am J Hematol. 2014;89(9):E150–E155. doi: 10.1002/ajh.23767. [DOI] [PubMed] [Google Scholar]
- 12.Naithani R, Agrawal N, Mahapatra M, Pati H, Kumar R, Choudhary VP. Autoimmune hemolytic anemia in India: clinico-hematological spectrum of 79 cases. Hematology. 2006;11(1):73–76. doi: 10.1080/10245330500345587. [DOI] [PubMed] [Google Scholar]
- 13.Bass GF, Tuscano ET, Tuscano JM. Diagnosis and classification of autoimmune hemolytic anemia. Autoimmun Rev. 2014;13(4–5):560–564. doi: 10.1016/j.autrev.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Barcellini W, Fattizzo B, Zaninoni A, Radice T, Nichele I, Di Bona E, Lunghi M, Tassinari C, Alfinito F, Ferrari A, Leporace AP, Niscola P, Carpenedo M, Boschetti C, Revelli N, Villa MA, Consonni D, Scaramucci L, De Fabritiis P, Tagariello G, Gaidano G, Rodeghiero F, Cortelezzi A, Zanella A. Clinical heterogeneity and predictors of outcome in primary autoimmune hemolytic anemia: a GIMEMA study of 308 patients. Blood. 2014;124(19):2930–2936. doi: 10.1182/blood-2014-06-583021. [DOI] [PubMed] [Google Scholar]
- 15.Barcellini W, Zaninoni A, Fattizzo B, Giannotta JA, Lunghi M, Ferrari A, Leporace AP, Maschio N, Scaramucci L, Cantoni S, Chiurazzi F, Consonni D, Rossi G, De Fabritiis P, Gaidano G, Zanella A, Cortelezzi A. Predictors of refractoriness to therapy and healthcare resource utilization in 378 patients with primary autoimmune hemolytic anemia from eight Italian reference centers. Am J Hematol. 2018;93(9):E243–E246. doi: 10.1002/ajh.25212. [DOI] [PubMed] [Google Scholar]
- 16.Alonso HC, Manuel AV, Amir CG, Sergio RR, Allan P, Xavier LK, Juventina TE. Warm autoimmune hemolytic anemia: experience from a single referral center in Mexico City. Blood Res. 2017;52(1):44–49. doi: 10.5045/br.2017.52.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van De Loosdrecht AA, Hendriks DW, Blom NR, Smit JW, De Wolf JT, Vellenga E. Excessive apoptosis of bone marrow erythroblasts in a patient with autoimmune haemolytic anaemia with reticulocytopenia. Br J Haematol. 2000;108(2):313–315. doi: 10.1046/j.1365-2141.2000.01867.x. [DOI] [PubMed] [Google Scholar]
- 18.Berentsen S. Role of complement in autoimmune hemolytic anemia. Transfus Med Hemother. 2015;42(5):303–310. doi: 10.1159/000438964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dussadee K, Taka O, Thedsawad A, Wanachiwanawin W. Incidence and risk factors of relapses in idiopathic autoimmune hemolytic anemia. J Med Assoc Thail. 2010;93(Suppl 1):S165–S170. [PubMed] [Google Scholar]
- 20.Yilmaz F, Vural F. Autoimmune hemolytic anemia: focusing on therapy according to classification. SOJ Immunol. 2017;5(1):1–6. [Google Scholar]
- 21.Kalfa TA. Warm antibody autoimmune hemolytic anemia. Hematol Am Soc Hematol Educ Program. 2016;2016(1):690–697. doi: 10.1182/asheducation-2016.1.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bussone G, Ribeiro E, Dechartres A, Viallard JF, Bonnotte B, Fain O, Godeau B, Michel M. Efficacy and safety of rituximab in adults’ warm antibody autoimmune haemolytic anemia: retrospective analysis of 27 cases. Am J Hematol. 2009;84(3):153–157. doi: 10.1002/ajh.21341. [DOI] [PubMed] [Google Scholar]