Figure 1.
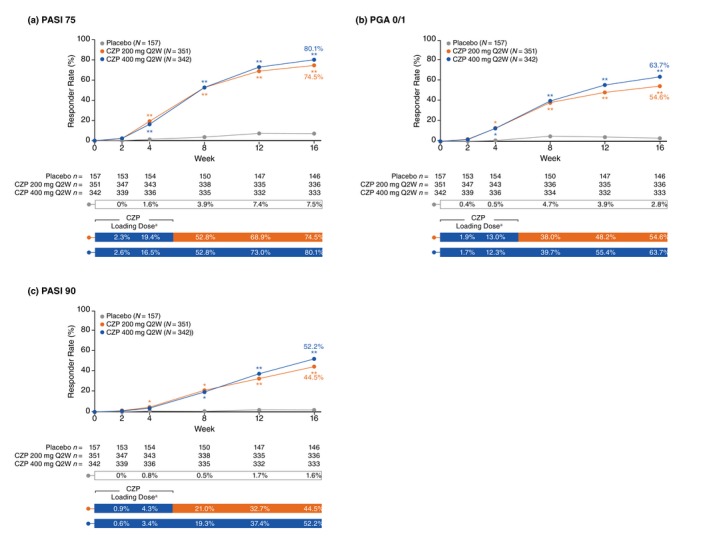
Pooled responder rates from baseline to week 16 (randomized set). *P < 0.05, **P < 0.0001 versus placebo (not adjusted for multiplicity). aCertolizumab pegol (CZP) 200 mg Q2W patients received loading dose of CZP 400 mg at Weeks 0, 2 and 4. Randomized set includes all randomized patients. Responder rates were analysed using a logistic regression model with factors of treatment, region, study, prior biologic exposure (yes/no), and the interactions study × region and study × prior biologic exposure (yes/no); Markov chain Monte Carlo methodology for multiple imputation was used to account for missing data. CZP, certolizumab pegol; PASI 75, ≥75% reduction in psoriasis area and severity index; PASI 90, ≥90% reduction in psoriasis area and severity index; PGA 0/1, ‘clear’/‘almost clear’ with ≥2‐category improvement in physician's global assessment; Q2W, every 2 weeks.
