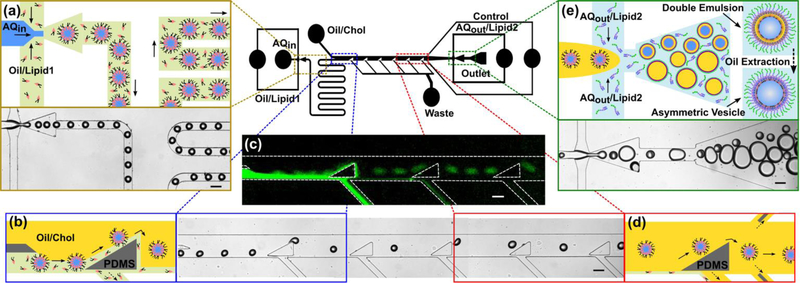
Fig. 1.
Schematics of our microfluidic technique that is capable of generating 50 to 150 μm diameter water-in-oil-in-water double emulsions at high-throughput. (a) Initially, uniform water-in-oil emulsions are made at the first flow focusing region. The phospholipid molecules (i.e. lipid-1) that are present in the oil phase (i.e. green domain) spontaneously assemble on the water-oil interface and proceed along the serpentine channel. (b) After the serpentine channel, a series of triangular posts force the emulsions to move across the two oil phases (i.e. green and yellow domains) and continue along the streamlines in the OL2. (c) The fluorescent image confirms the assembly of NBD-tagged phospholipids on the emulsions and their separation from the OL1 stream (i.e. green flow). The OL1 stream goes into the waste channels located under the triangular posts. (d) Finally, the water-in-oil emulsions, covered with a monolayer of lipid-1 molecules, approach the second flow focusing region. (e) The emulsions are encapsulated in an ultrathin OL2 layer containing cholesterol and form water-in-oil-in-water (w/o/w) double emulsions dispersed in the OA domain in which the LPS molecules are dissolved. Ethanol was introduced to the OA domain in order to extract the intermediate oil layer from the double emulsions and to bring the two lipid monolayers together. Flow rates of the IA, OL1, OL2, and OA streams were set at 12, 22, 400, and 380 μL h−1, respectively. All scale bars denote 100 μm.