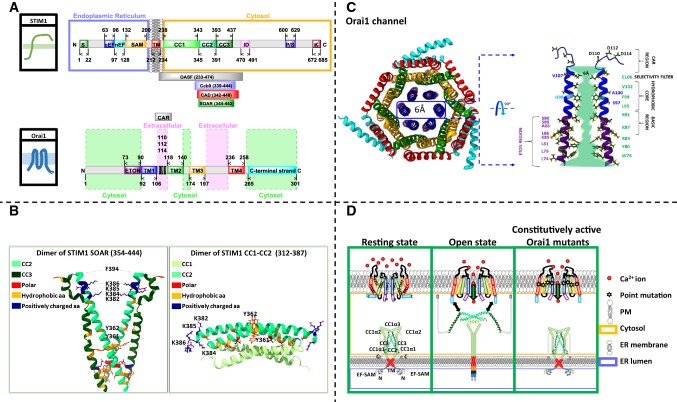
Fig. 1.
Basic features on STIM1 and Orai1. A Scheme showing the critical regions within full-length human STIM1 and Orai1 that regulate their interplay. Important STIM1 fragments, as OASF, Ccb9, CAD, and SOAR are further represented as insets. B The X-ray structure of STIM1 SOAR dimer (left) and the NMR structure consisting of a dimer of STIM1 CC1α3–CC2 monomers (right). STIM1 SOAR dimers form a V-shape and consist of CC2 and CC3 domains. Each monomer resembles the capital letter “R”. Essential residues that mediate STIM1–STIM1 or STIM1–Orai1 interactions and that are described in the review are highlighted. The STIM1 NMR structure shows that STIM1 CC1α3–CC2 monomers couple in an antiparallel manner. Each monomer exhibits a sharp kink between the two coiled–coil domains. C The scheme shows the hexameric assembly of human Orai1 according to the Drosophila dOrai crystallography structure. The inner ring surrounding the pore is formed by TM1, while the other TM domains within the 6 subunits are arranged into two concentric rings around the pore (left). The cartoon shows two opposite TM1 domains with the cytosolic and extracellular helical extension and represents the important residues lining the pore. The pore can be separated into the CAR region, the selectivity filter, the hydrophobic core, the basis region and the ETON region. D The scheme shows STIM1 and Orai1 in the resting state, how Orai channel activation occurs via STIM1 and how Orai channel becomes constitutively active upon single-point mutation independent of STIM1