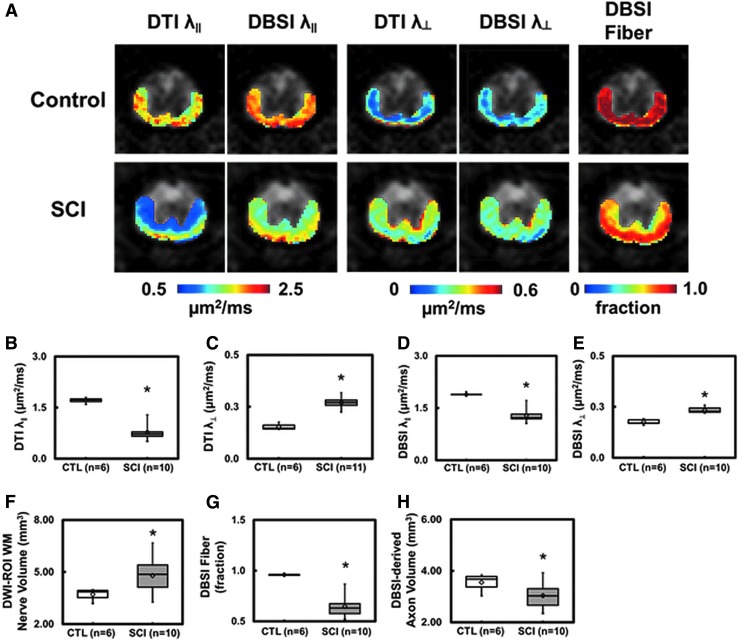
FIG. 1.
Representative in vivo diffusion tensor imaging (DTI) and diffusion basis spectrum imaging (DBSI) metric maps were overlaid on gray-scale diffusion-weighted images from one control and one spinal cord injury (SCI) mouse at T9 vertebral level (A). DTI axon/myelin pathological metrics are susceptible to the effect of co-existing inflammation and axonal loss that could exaggerate or underestimate the severity of SCI. In the present study, inflammatory cell infiltration resulted in a more significantly decreased DTI axial diffusivity (λ∥) (B) than that derived by DBSI (D), while the combined axonal loss and vasogenic edema led to a more significantly increased DTI radial diffusivity (λ⊥) (C) than DBSI λ⊥ (E). Increased white matter volume (F; as a result of cell infiltration and vasogenic edema) and decreased DBSI fiber fraction (G; the decreased axonal density as a result of combined effects of increased cell infiltration, vasogenic edema, and axonal loss) were present at 3 days after SCI. DBSI-derived axonal volume (H) derived as the product of white matter volume (F) times DBSI fiber fraction (G) reflects the extent of axonal loss in the presence of tissue swelling. At this time-point, decreased DBSI-derived apparent axonal volume was observed, suggesting axonal loss (H). The intensity gradients in DBSI λ∥, λ⊥, and fiber fraction maps reflect spatial variation of injury severity that are intrinsic to the dorsal-to-ventral impact nature of this SCI model. *p < 0.05.