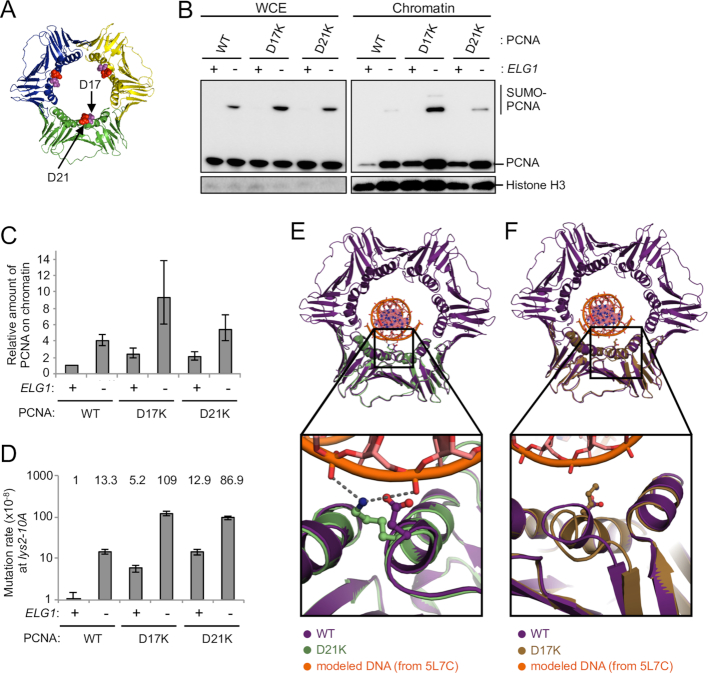
Figure 2.
Identification of new PCNA mutants that are retained on DNA due to enhanced electrostatic interaction and cause increase in mutation rate. (A) Structure of the PCNA trimer (PDB ID: 3K4X) (51). The positions mutated are highlighted. (B) The inner ring surface PCNA mutants (D17K and D21K) accumulate on chromatin. WCE and chromatin-enriched fractions (Chromatin) were prepared from cells expressing PCNA mutants in log phase. The PCNA mutants are the only copy of PCNA in these cells. PCNA and histone H3 (loading control) were detected by Western blotting. (C) Quantification of chromatin-bound PCNA and PCNA mutants. Average of two experiments of total PCNA on chromatin (normalized to histone H3), relative to WT, is shown. Error bars, SDs. (D) Mutation rates of ELG1+ and elg1Δ in the wild-type and retention-prone PCNA mutants backgrounds at the lys2–10A locus. Fold increases over wild-type are shown above. Error bars, 95% confidence intervals. (E) 2.8 Å resolution crystal structure of PCNA-D21K (green, PDB ID: 6D0Q) compared with a hybrid model of WT yeast PCNA (purple, PDB ID: 4YHR) with DNA modeled based on a human PCNA:DNA cocrystal structure (PDB ID: 5L7C). Inset shows that lysine 21 could simultaneously interact with two DNA phosphate groups. (F) 2.85 Å crystal structure of PCNA-D17K (tan, PDB ID: 6D0R) compared with a hybrid model of WT yeast PCNA (purple, PDB ID: 4YHR) bound to DNA (PDB ID: 5L7C). Inset shows that lysine 17 is positioned within the major groove of DNA, but also induces a conformational change helix 1 and an adjacent loop.