Table 5. Optimization of the Reaction Conditions for the β-Alkylation of Secondary Alcoholsa.
| entry | cat. 1 (mol %) | base (mol %) | conv. (%)b | yield (%)c |
|---|---|---|---|---|
| 1 | 3 | Cs2CO3 (10) | >99 | 80 |
| 2 | 2 | Cs2CO3 (10) | >99 | 88 |
| 3d | 1 | Cs2CO3 (10) | 87 | 54 |
| 4 | 2 | Cs2CO3 (5) | >99 | 90 |
| 5d | 1 | Cs2CO3 (5) | 37 | 23 |
| 6d | 2 | Cs2CO3 (2.5) | 51 | 42 |
| 7 | 2 | 5 | 0 | |
| 8 | Cs2CO3 (5) | 15 | 0 | |
| 9 | 0 | 0 |
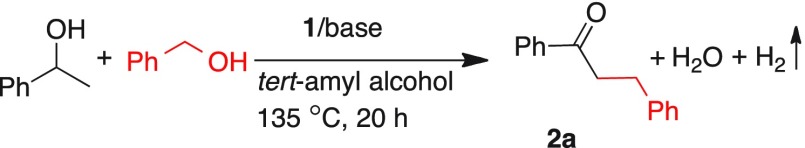
Reaction conditions: 1-phenyl-1-ethanol (0.5 mmol), benzyl alcohol (0.6 mmol), tert-amyl alcohol (2 mL), catalyst 1, and base were heated at 135 °C with open condition under an argon flow.
Conversion of 1-phenyl-1-ethanol was determined by GC using toluene as an internal standard.
Isolated yields after column chromatography.
A minor amount of (E)-chalcone formation also observed.