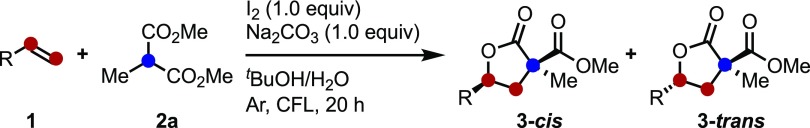
Table 2. trans-Diastereoselective Synthesis of Lactones, 3, from Various Alkenes Using Malonate 2a.
| entry | R | 3 | yield (%) | dra (cis:trans) |
|---|---|---|---|---|
| 1 | Ph (1a) | 3aa | 69 | 25:75 |
| 2 | 4-tBu-C6H4 (1b) | 3ba | 65 | 33:67 |
| 3 | 4-Me-C6H4 (1c) | 3ca | 61 | 33:67 |
| 4 | 3-Me-C6H4 (1d) | 3da | 72 | 33:67 |
| 5 | 2-Me-C6H4 (1e) | 3ea | 66 | 26:74 |
| 6 | 4-F-C6H4 (1f) | 3fa | 28 | 20:80 |
| 7 | 4-Cl-C6H4 (1g) | 3ga | 60 | 29:71 |
| 8 | 4-Br-C6H4 (1h) | 3ha | 75 | 26:74 |
| 9 | 4-MeO2C-C6H4 (1i) | 3ia | nd | |
| 10 | 4-MeO-C6H4 (1j) | 3ja | trace | |
| 11 | 4-Ph-C6H4 (1k) | 3ka | 38 | 33:67 |
| 12 | 2-naph (1l) | 3la | 75 | 28:72 |
| 13 | 2-pyridyl (1m) | 3ma | 50 | 37:63 |
| 14 | C10H21 (1n) | 3na | 50 | 33:67 |
Diasteromeric ratios (dr) were determined by 1H NMR analysis of the crude reaction mixture.