Figure 1.
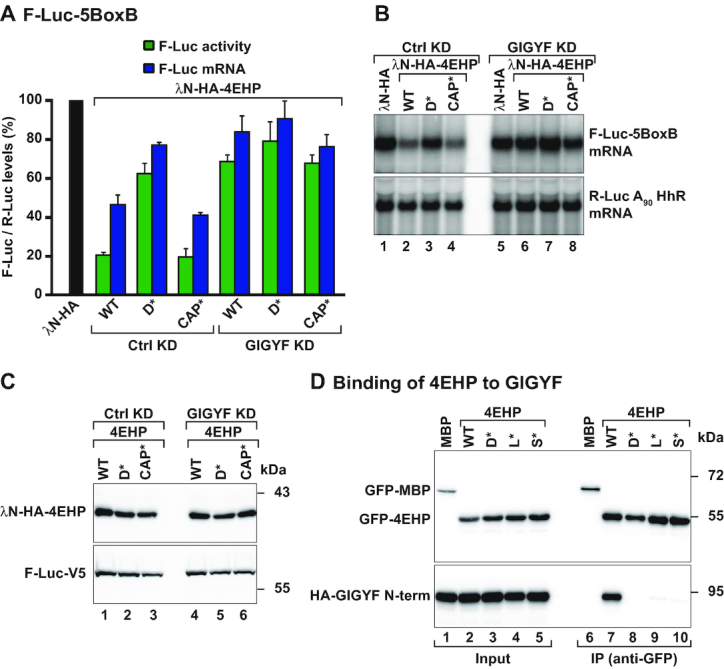
Dm 4EHP requires Dm GIGYF to downregulate reporter mRNA expression. (A) Tethering assay using the firefly luciferase (F-Luc)-5BoxB reporter and the indicated λN-HA-tagged proteins in control [Ctrl knockdown (KD): neomycin dsRNA-treated cells] or GIGYF-depleted Dm S2 cells (GIGYF KD). A plasmid expressing Renilla luciferase (R-Luc)-A90-HhR served as a transfection control. The F-Luc activity (green bars) and mRNA levels determined by northern blotting (blue bars) were normalized to those of the R-Luc transfection control and set to 100% in cells expressing the λN-HA peptide (black bar). Bars represent the mean values and error bars denote the standard deviation from at least three independent experiments. (B) Northern blot analysis of a representative tethering experiment shown in (A). (C) Western blot showing the expression levels of the tethered proteins. (D) Immunoprecipitation assay showing the interaction between GFP-tagged 4EHP WT or the indicated mutants (dorsal: D*; lateral: L*; and 4EHP-specific: S*) and HA-tagged GIGYF N-term. The proteins were immunoprecipitated using an anti-GFP antibody. GFP-MBP (maltose binding protein) served as a negative control. The input (2.8% of the total lysate) and bound fractions (10%) were analyzed by western blotting using anti-GFP and anti-HA antibodies.
