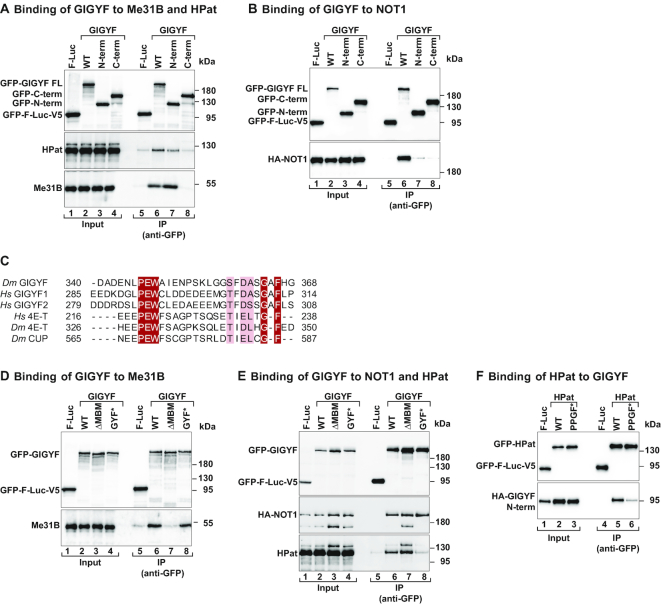
Figure 3.
Dm GIGYF interacts with components of the decapping and deadenylation machineries. (A) Immunoprecipitation assay showing the interaction between GFP-GIGYF full length (WT), and the indicated fragments, and endogenous HPat and Me31B. The proteins were immunoprecipitated in the presence of RNaseA using an anti-GFP antibody. GFP-F-Luc-V5 served as a negative control. The input (3% for GFP-tagged proteins, 0.2% for HPat and 0.4% for Me31B) and bound fractions (30% for GFP-tagged proteins and HPat, and 40% for Me31B) were analyzed by western blotting using anti-GFP, anti-HPat and anti-Me31B antibodies. (B) The interaction among full length (WT) and fragments of GFP-GIGYF and HA-NOT1 was determined by anti-GFP immunoprecipitation in S2 cell lysates. GFP-F-Luc-V5 served as a negative control. The input (3% for GFP-tagged proteins and 1% for HA-tagged proteins) and bound fractions (15% for GFP-tagged proteins and 30% for HA-tagged proteins) were analyzed by western blotting using anti-GFP and anti-HA antibodies. (C) Sequence alignment of the MBM present in Drosophila melanogaster (Dm) GIGYF and human (Hs) GIGYF1 and 2 proteins with the CUP-homology region from the Hs and Dm 4E-T proteins or the Dm CUP protein. Identical residues are highlighted in red boxes and printed in white whereas residues with >70% similarity are shown with a light color background. (D) Immunoprecipitation assay showing the interaction between GFP-tagged GIGYF proteins (WT and indicated mutants) and endogenous Me31B. The proteins were immunoprecipitated using anti-GFP antibodies. GFP-F-Luc-V5 served as a negative control. The input (4% for GFP-tagged proteins and 0.18% for endogenous Me31B) and bound fractions (40% for GFP-tagged proteins and 45% for endogenous Me31B) were analyzed by western blotting using anti-GFP, anti-Me31B antibodies. (E) The interaction of HA-NOT1 and endogenous HPat with WT and mutant GIGYF proteins was analyzed by immunoprecipitation using anti-GFP antibodies. GFP-F-Luc-V5 served as a negative control. The input (4% for GFP-tagged proteins and 0.8% for HA-tagged proteins and endogenous HPat) and bound fractions (15% for GFP-tagged proteins and 35% for HA-NOT1 and endogenous HPat) were analyzed by western blotting using anti-GFP, anti-HA and anti-HPat antibodies. (F) Western blot analysis of the interaction of GFP-HPat [WT and PPGF motif mutant (PPGF*)] with HA-GIGYF N-term. The input (2% for GFP-tagged proteins and 0.8% for HA-tagged proteins) and bound fractions (10% for GFP-tagged proteins and 20% for HA-tagged proteins) were analyzed by western blotting using anti-GFP and anti-HA antibodies.