ABSTRACT
Background
Elevated gamma‐glutamyl transferase (GGT) levels have been demonstrated to be associated with poor prognoses in patients with coronary artery disease. Coronary computed tomography angiography (CCTA) is a noninvasive imaging modality that may differentiate the structure of coronary plaques. Elevated plaque burdens and noncalcified plaques, detected by CCTA, are important predictors of atherosclerosis in young adults.
Hypothesis
The present study investigated the possible relationship between GGT levels and coronary plaque burdens/structures in young adults with coronary atherosclerosis.
Methods
CCTA images of 259 subjects were retrospectively examined, and GGT levels were compared between patients with coronary plaques and individuals with normal coronary arteries. Coronary plaques, detected by CCTA, were categorized as noncalcified, calcified, and mixed, according to their structures. The significant independent predictors of coronary atherosclerosis were also analyzed using multivariate logistic regression analysis.
Results
GGT levels were significantly higher in patients with coronary plaque formation than in controls (35.7 ± 14.7 vs 19.6 ± 10.0 U/L; P < 0.001). GGT levels were also positively correlated with the number of plaques; presence of noncalcified plaques; and levels of high‐sensitivity C‐reactive protein (hs‐CRP), hemoglobin A1c, uric acid, and triglycerides. Moreover, smoking and levels of GGT, hs‐CRP, uric acid, and low high‐density lipoprotein cholesterol were independent predictors of coronary atherosclerosis.
Conclusions
GGT is an inexpensive and readily available marker that provides additional risk stratification beyond that provided by conventional risk factors for predicting coronary plaque burdens and plaque structures in young adults.
Introduction
Globally, cardiovascular diseases are among the leading causes of morbidity and mortality. The incidence of symptomatic coronary artery disease (CAD) at a young age is low, involving approximately 3% of all CAD cases.1 Although CAD is rarely seen in adults younger than 45 years of age, identification of risk factors and early diagnoses are important, as symptoms may indicate unexpected and serious consequences. Atherosclerotic changes are well‐known to begin early in life, and the presence of additional risk factors, such as a genetic predisposition, obesity, dyslipidemia, high blood pressure, and smoking may accelerate the disease progress, resulting in acute coronary syndromes.2
Noninvasive imaging modalities, such as carotid intima‐media thickness measurements and coronary artery calcium (CAC) score calculations, have gained attention in the diagnosis of subclinical atherosclerosis and risk predictions for future adverse cardiovascular events.3, 4 Coronary computed tomography (CT) angiography (CCTA), which is a noninvasive imaging technique with a high sensitivity and specificity for the diagnosis of CAD, may provide valuable information about an individual's plaque burden and plaque structure.5, 6, 7, 8
Gamma‐glutamyl transferase (GGT) measurement is a second‐generation hepatic function test that is used as an indicator of alcohol ingestion, hepatic inflammation/infection, and fatty liver disease. GGT levels are also associated with the presence and severity of atherosclerosis, and also may be a risk factor for future cardiovascular events.9, 10, 11, 12 However, the exact pathophysiological relationship between coronary atherosclerosis and GGT remains unclear, especially in young adults.
To our knowledge, there are no studies regarding the association between GGT levels and coronary plaque burdens/structures in young adults with coronary atherosclerosis. This study investigated this relationship using CCTA in patients under 45 years old.
Methods
Patient Selection
This single‐center, retrospective, case‐control study was conducted at a tertiary heart center. The CCTA examinations performed between January 2011 and November 2013 were screened, and 410 patients under 45 years of age were identified. The indications for CCTA in the study population were patients with atypical chest pain, but at intermediate risk for CAD; inconclusive or uninterpretable stress test results; evaluation of suspected coronary anomalies; and exclusion of significant CAD before noncoronary cardiac surgery. The exclusion criteria were prior diagnosis of CAD, coronary revascularization procedures, heart failure, valvular heart disease, aortic aneurysms, renal dysfunction, hepatitis B or C infection, other known liver diseases, hemolytic disorders, acute/chronic inflammatory conditions, neoplastic diseases, use of alcohol (all alcohol drinkers) and hepatotoxic drugs, and missing laboratory parameters. In addition, abdominal ultrasonography was performed to exclude the presence of any underlying liver disease, including hepatosteatosis. After the initial evaluation, 151 subjects were excluded from further analysis. Finally, a total of 259 patients were included in the study (Figure 1). Demographic information, cardiovascular risk factors (age, family history, smoking habit, hyperlipidemia, body mass index, hypertension, and diabetes mellitus) were recorded following a systematic review of the hospital records. Missing variables were obtained by telephone interviews with the patient and/or relatives. Informed consent was obtained from each subject, and the study protocol was approved by the local ethics committee.
Figure 1.
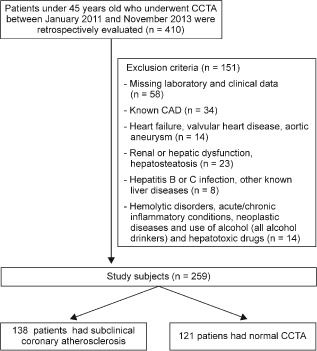
Selection of study participants. Abbreviations: CAD, coronary artery disease; CCTA, coronary computed tomography angiography.
Definitions
The data regarding the daily smoking habits of current smokers were obtained. Past smokers, included in the smoker category, were defined as those who had abstained from smoking for >3 months at the time of the examination. Hypertension was diagnosed if systolic arterial pressure exceeded 140 mm Hg and/or diastolic arterial pressure exceeded 90 mm Hg, or if the patient was using antihypertensive drugs.13 Diabetes mellitus was diagnosed if fasting glucose levels exceeded 126 mg/dL or if the patient used prescribed glucose‐lowering agents.14 Hyperlipidemia was defined as total serum cholesterol levels >240 mg/dL, low‐density lipoprotein (LDL)‐cholesterol >130 mg/dL, or serum triglycerides >180 mg/dL, or if the patient used lipid‐lowering drugs.15
CCTA Examination
CCTA images were obtained using a dual‐source CT system (Definition Flash; Siemens Medical Solutions, Forchheim, Germany) with 280 ms of rotation time, 2 × 128 slices, a pitch of 3.4, and triggering at 60% of the R‐R interval. The tube current was set at 180‐300 mAs, and a 0.6‐mm slice collimation was achieved. Nonionic contrast material (Iomeron® 400 mg/mL; Bracco, Milan, Italy) at a dose of 80 to 100 mL was administered at a rate of 5 mL/s, with a dual‐head power injector attached to an 18‐gauge needle positioned in an antecubital vein. The bolus tracking technique was used and images were obtained during a single, 6‐second breath‐hold.
Image Analysis
Two experienced radiologists, blinded to the clinical properties of the subjects, analyzed the scans on a 3‐dimensional workstation (Syngo; Siemens Healthcare, Erlangen, Germany). A final diagnosis using multidetector CT was achieved by consensus. The radiologists analyzed the characteristics of the stenosis and the number of plaques/segment based on the modified American Heart Association classification.16, 17 Stenosis >50% was considered significant and >75% was defined as severe.
Plaques were defined as 1‐mm2 structures, within or adjacent to a vessel lumen, which could be clearly distinguished from the lumen and the surrounding pericardial tissue. Coronary plaques were classified as noncalcified, calcified, and mixed according to their structure. Plaques without any calcification were defined as noncalcified, plaques with >50% of the plaque area occupied by calcified tissue (density ≥130 Hounsfield in native scans) were defined as calcified, and plaques with <50% calcium were defined as a mixed type.16 The plaque burden was calculated as the sum of the atherosclerotic segments (1 point for each) in the coronary arteries, which were divided into 15 segments.17
Laboratory Analyses
Venous blood samples were drawn for the analysis of hemoglobin A1c (HbA1c), high‐sensitivity C‐reactive protein (hs‐CRP), total cholesterol, triglycerides, uric acid, and serum creatinine levels after a minimum 12‐hour fasting period (Cobas C501 Autoanalyzer; Roche Diagnostics, Mannheim, Germany). Serum creatinine levels were measured using the alkaline picrate (Jaffe) method, and hs‐CRP was analyzed turbidimetrically. Serum GGT levels were measured using an enzymatic colorimetric test with a Roche/Cobas analyser at 37°C and L‐gamma‐glutamyl‐3‐carboxy‐4‐nitroanilide was used as a substrate (Cobas C501 Autoanalyzer; Roche Diagnostics). The normal GGT reference value for a healthy individual was 7 to 49 U/L in our laboratory.
Statistical Analysis
Descriptive statistics were expressed as numbers (%) for categorical variables and as mean, median, standard deviation, minimum, and maximum for numerical variables. The normal distribution of continuous variables was checked using the Kolmogorov‐Smirnov test. Differences between patients and controls were evaluated using 2‐sample t tests, analysis of variance, and the Mann‐Whitney U test, as appropriate. A χ2 test was used to compare independent categorical variables. Relationships between numerical variables were identified using Pearson or Spearman correlation tests, and a receiver operator characteristic (ROC) curve analysis was used to determine GGT cutoff values for the diagnosis of coronary atherosclerosis. In addition, backward, stepwise, multivariate logistic regression analyses, which included variables with P values <0.10 in the univariate analysis, were performed to identify the independent predictors of coronary atherosclerosis; a P value <0.05 was considered statistically significant. Data were analyzed using the Statistical Package for the Social Sciences version 16.0 (SPSS, Inc., Chicago, IL).
Results
The present study included 138 patients with coronary atherosclerosis (mean age, 41.6 ± 3.0 years; 72.5% male) and 121 control subjects (mean age, 41.9 ± 3.3 years; 64.5% male). Baseline demographic, clinical, and laboratory characteristics of the study groups are summarized in Table 1. Age, gender, body mass index, and family histories of CAD were not statistically different between the 2 groups. However, the frequencies of smoking, diabetes mellitus, and hypertension were higher in the CAD group than in the controls. In the CAD patients, the mean number of diseased vessels per patient was 1.7 ± 0.8, and the total number of plaques was 2.1 ± 1.4. Although the number of noncalcified plaques was highest (1.2 ± 1.0), the numbers of calcified and mixed types were similar (0.8 ± 0.5 and 0.7 ± 0.4, respectively).
Table 1.
Baseline Demographic, Clinical, and Laboratory Characteristics of the Study Groups
| Patients, n = 138 | Controls, n = 121 | P | |
|---|---|---|---|
| Age, y | 41.6 ± 3.0 | 41.9 ± 3.3 | 0.20 |
| Gender | |||
| Male, n (%) | 100 (72.5) | 78 (64.5) | 0.17 |
| Female, n (%) | 38 (27.5) | 43 (35.5) | |
| BMI, kg/m2 | 30.03 ± 4.84 | 28.78 ± 3.95 | 0.10 |
| Family history, n (%) | 54 (39.1) | 57 (47.1) | 0.20 |
| Smoking, n (%) | 98 (71.4) | 38 (31.4) | 0.01 |
| Diabetes mellitus, n (%) | 32 (23.2) | 15 (12.4) | 0.02 |
| Hypertension, n (%) | 56 (40.6) | 32 (26.4) | 0.02 |
| Hyperlipidemia, n (%) | 58 (42) | 30 (24.8) | 0.01 |
| CCTA findings | |||
| No. of diseased vessels | 1.7 ± 0.8 | — | — |
| No. of plaques | 2.1 ± 1.4 | — | — |
| Distribution of plaques | |||
| Calcified type | 0.8 ± 0.5 | — | — |
| Noncalcified type | 1.2 ± 1.0 | — | — |
| Mixed type | 0.7 ± 0.4 | — | — |
| Laboratory findings | |||
| Creatinine, mg/dL | 0.71 ± 0.13 | 0.68 ± 0.16 | 0.01 |
| Total cholesterol, mg/dL | 216.5 ± 43.5 | 200.2 ± 39.3 | 0.01 |
| LDL‐cholesterol, mg/dL | 144.6 ± 40.6 | 129.8 ± 35.1 | 0.01 |
| HDL‐cholesterol, mg/dL | 34.8 ± 6.6 | 44.2 ± 11.6 | 0.01 |
| Triglyceride | 187.6 ± 123.6 | 140.8 ± 90.1 | 0.01 |
| Uric acid, mg/dL | 5.8 ± 1.2 | 4.7 ± 1.07 | 0.01 |
| HbA1c, % | 6.2 ± 1.3 | 5.8 ± 0.6 | 0.01 |
| hs‐CRP, mg/dL | 1.78 ± 0.72 | 1.04 ± 0.51 | 0.01 |
| GGT, U/L | 35.7 ± 14.7 | 19.6 ± 10.0 | 0.01 |
Abbreviations: BMI, body mass index; CCTA, coronary computed tomography angiography; GGT, gamma‐glutamyl transferase; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; hs‐CRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein.
Values are presented as means ± standard deviation or number (%), as appropriate.
Plasma creatinine, uric acid, HbA1c, hs‐CRP, total cholesterol, LDL cholesterol, and triglyceride levels were significantly higher in the patient group than in the controls (P = 0.01, for each comparison). However, the high‐density lipoprotein (HDL) cholesterol levels were significantly lower in the patient group (P = 0.01). The mean GGT level was significantly higher in the patient group than in the control group (35.7 ± 14.7 U/L vs 19.6 ± 10.0 U/L; P = 0.01). In the subgroup analysis, the GGT and hs‐CRP levels were significantly higher in subjects with ≥3 plaques (n = 38) compared with those with <3 plaques (n = 100) (43.1 ± 13.1 vs 32.9 ± 14.4 U/L, P = 0.01; and 2.03 ± 0.76 vs 1.67 ± 0.67 mg/L, P = 0.01; respectively). When the relationship between the GGT levels and coronary plaque structure was investigated in subjects having only 1 coronary artery plaque (n = 61), the GGT level was not statistically different between the noncalcified (n = 36), calcified (n = 16), and mixed (n = 9) subgroups (31.5 ± 16.1 vs 30.9 ± 13.2 vs 34.7 ± 18.5 U/L, respectively; P = 0.82).
Twenty‐three subjects (16.6%) from the CAD group and 14 subjects (11.6%) from the control group were administered statins (P = 0.26); 5 subjects (3.6%) from the CAD group and 2 subjects from the control group (1.7%) were using fenofibrate therapy (P = 0.45). In the subgroup analyses, when the subjects undergoing statin or fibrate treatment were excluded, GGT levels were still higher in the CAD group than in the control group (34.9 ± 14.5 U/L vs 20.1 ± 10.4 U/L, P = 0.01).
The discriminatory value of GGT levels for coronary atherosclerosis was assessed by ROC curve analysis and revealed a sensitivity (71.7%), specificity (80.2%), positive predictive value (71.3%), and negative predictive value (80.5%) of a cutoff value of >26 U/L (area under the curve, 0.823; 95% confidence interval [CI]: 0.773‐0.874; P < 0.001).
In a univariate correlation analysis, the GGT level was positively correlated with the number of diseased vessels and the total number of plaques and levels of hs‐CRP, HbA1c, uric acid, and triglycerides, and negatively correlated with HDL‐cholesterol levels (Table 2). Moreover, the number of diseased vessels and the total number of coronary plaques were positively correlated with the hs‐CRP and HbA1c levels (P = 0.01, for each correlation).
Table 2.
The Correlation Analysis Between Gamma‐Glutamyl Transferase Levels and Coronary Artery Disease Risk Factors
| r | P | |
|---|---|---|
| No. of diseased vessels | 0.324 | 0.01 |
| No. of plaques | 0.335 | 0.01 |
| hs‐CRP | 0.572 | 0.01 |
| HbA1c | 0.199 | 0.02 |
| Uric acid | 0.321 | 0.01 |
| Total cholesterol | 0.069 | 0.42 |
| LDL‐cholesterol | 0.025 | 0.77 |
| HDL‐cholesterol | −0.383 | 0.01 |
| Triglyceride | 0.271 | 0.01 |
Abbreviations: HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; hs‐CRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein.
In a univariate regression analysis, diabetes mellitus; hypertension; smoking; and elevated levels of GGT, hs‐CRP, uric acid, creatinine, total cholesterol, LDL‐cholesterol, and triglycerides were significantly associated with coronary atherosclerosis, as were decreased HDL‐cholesterol levels (Table 3). Smoking and levels of GGT, hs‐CRP, uric acid, HDL‐cholesterol, and triglycerides were independent predictors of coronary atherosclerosis, after adjusting for other risk factors (Table 4).
Table 3.
Univariate Analysis for Predictors of Coronary Atherosclerosis
| OR | CI | P | |
|---|---|---|---|
| Gender | 1.451 | 0.856‐2.458 | 0.167 |
| Age | 1.092 | 0.995‐1.200 | 0.065 |
| Diabetes mellitus | 2.13 | 1.092‐4.168 | 0.027 |
| Hypertension | 1.899 | 1.120‐3.220 | 0.017 |
| Smoking | 5.531 | 3.245‐9.106 | <0.001 |
| GGT | 1.110 | 1.080‐1.140 | <0.001 |
| hs‐CRP | 9.076 | 4.904‐16.797 | <0.001 |
| Creatinine | 5.627 | 0.999‐31.685 | 0.050 |
| Uric acid | 2.382 | 1.815‐3.127 | <0.001 |
| Total cholesterol | 1.010 | 1.003‐1.016 | 0.002 |
| LDL‐cholesterol | 1.011 | 1.004‐1018 | 0.003 |
| HDL‐cholesterol | 0.885 | 0.857‐0.919 | <0.001 |
| Triglyceride | 1.005 | 1.002‐1.008 | 0.001 |
Abbreviations: CI, confidence interval; GGT, gamma‐glutamyl transferase; HDL, high‐density lipoprotein; hs‐CRP; high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein; OR, odds ratio.
Table 4.
Multivariate Logistic Regression Analysis for Independent Predictors of Coronary Atherosclerosis
| OR | CI | P | |
|---|---|---|---|
| Smoking | 3.027 | 1.738‐6.651 | 0.006 |
| GGT | 1.056 | 1.015‐1.098 | 0.006 |
| hs‐CRP | 4.843 | 2.004‐11.705 | <0.001 |
| Uric acid | 1.894 | 1.270‐2.825 | 0.002 |
| HDL‐cholesterol | 0.915 | 0.866‐0.967 | 0.002 |
Abbreviations: CI, confidence interval; GGT, gamma‐glutamyl transferase; HDL, high‐density lipoprotein; hs‐CRP; high‐sensitivity C‐reactive protein; OR, odds ratio.
Discussion
The main findings of the present study were that (1) GGT levels were significantly higher in individuals with coronary atherosclerosis under 45‐years‐old; (2) GGT levels correlated with the total number of coronary plaques and were highest in subjects with ≥3 plaques; and (3) smoking and levels of GGT, hs‐CRP, uric acid, HDL‐cholesterol, and triglycerides were independent predictors of coronary atherosclerosis. These findings indicated that elevated GGT levels may implicate the presence of atherosclerotic changes and may suggest risk beyond that described by well‐defined cardiovascular risk factors.
GGT measurements are low‐cost, simple, and highly sensitive indicators that provide an index of hepatobiliary dysfunction. The relationship between high GGT levels with well‐known cardiovascular risk factors, such as obesity, high blood pressure, unfavorable plasma lipid profiles, diabetes or insulin resistance, and decreased physical activity have been reported in previous studies.18, 19, 20, 21 Additionally, the prognostic value of raised GGT levels has been presented in patients with cardiovascular disease.22 In this study, GGT levels were correlated with plaque burden and structure, as detected by CCTA, in patients without established CAD. Moreover, elevated GGT levels were significant independent predictors of coronary atherosclerosis in young adults.
GGT activity has been detected in the atheromatous plaques of the carotid and coronary arteries, where it colocalizes with oxidized LDL.23 Serum GGT, within its high reference range, is an early and sensitive enzyme related to oxidative stress and plays a pivotal role in the pathogenesis of atherosclerosis.24 The degradation of the extracellular antioxidant, glutathione, by GGT enhances the body's pro‐oxidant status and the production of reactive oxygen species that result in atherosclerotic changes.25 Additionally, GGT catalysis of LDL oxidation, in atherosclerotic plaques, may contribute to plaque evolution and instability.23, 24, 25, 26 In support of these mechanisms, GGT levels were significantly correlated with inflammatory marker levels and the total plaque numbers in young adults with coronary atherosclerosis. The correlation between GGT levels and subclinical atherosclerosis has also been shown using imaging modalities, such as CAC and intima‐media thickness measurements.27, 28, 29 Lee et al conducted a study in 14 439 adults without known CAD and reported an independent association between increased serum GGT levels and the frequency of CAC.29 However, Bian et al found that GGT levels were only associated with the incidence of CAC scores (>100) in women.30 Even though the exact relationship between GGT levels and atherosclerosis has not been fully elucidated, chronic inflammation, oxidative stress, and lipid metabolism abnormalities may contribute to the mechanism.
Nevertheless, a CAC score of 0 does not guarantee the absence of coronary atherosclerosis; CCTA imaging may show noncalcified plaques (NCPs) in patients without calcium deposition.31, 32, 33 In 12.4% of patients with a CAC score of 0, coronary plaques were detected, and in 1.6% of the cases the lesions were obstructive.34 Jin et al reported the rate of subclinical atherosclerosis in asymptomatic young adults to be 9.4%.35 Concordant with our findings, NCPs were the most frequent type of coronary plaque and were independent risk factors for subclinical coronary atherosclerosis in young adults in their study. Thus, in patients <45 years old, especially those with multiple risk factors, coronary atherosclerosis develops as NCPs. However, in our study, as more than 1 type of plaque existed concomitantly in the same subject, we could not find a statistically significant difference between the different types of plaques. However, as NCPs were the most frequent type of plaque, and GGT levels were significantly correlated with the number of plaques, we may assume that GGT levels may be correlated with the presence of NCPs. Furthermore, the presence of NCPs, detected by CCTA, was associated with higher numbers of CAD risk factors (particularly smoking, obesity, and hypertension) in patients with 0 CACs.33 Unstable NCPs cause positive arterial remodeling, which alters flow dynamics, heightens the surface tension on the plaque surface, and raises the vulnerability of coronary plaques, resulting in plaque rupture and acute coronary syndromes. Yoo et al highlighted the importance of NCPs, detected by CCTA, in asymptomatic patients with low CAC scores, and emphasized that CCTA was a beneficial technique for CAD risk stratification.36 Thus, the establishment of new parameters associated with the presence of NCPs may have clinical importance in the prevention of cardiovascular events in young adults. In our study, the correlation of serum GGT levels with coronary atherosclerosis was demonstrated in terms of plaque burden/structure rather than only CAC scoring, which provides more accurate and reliable information about the severity of coronary artery atherosclerosis in young adults.
Study Limitations
There are some limitations associated with the present study. First, this study involved a single center and was retrospective in design. Second, the absence of follow‐up data describing future cardiovascular events meant that the prognostic value of GGT levels was not evaluated. Third, the study participants were Turkish, so the results may not be applicable to other ethnic groups or populations.
Conclusion
We demonstrated a significant relationship between serum GGT levels and the total number of coronary plaques and the plaque structures, as detected by CCTA. In addition, GGT levels were found to be significant independent predictors of coronary atherosclerosis in young adults. GGT measurements are accurate, readily available, and cost‐effective tests that may help identify young adults potentially at risk of coronary atherosclerosis who may require early CAD intervention.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Jalowiec DA, Hill JA. Myocardial infarction in the young and in women. Cardiovasc Clin. 1989;20:197–206. [PubMed] [Google Scholar]
- 2. Juonala M, Viikari JS, Raitakari OT. Main findings from the prospective Cardiovascular Risk in Young Finns Study. Curr Opin Lipidol. 2013;24:57–64. [DOI] [PubMed] [Google Scholar]
- 3. Bauer M, Caviezel S, Teynor A, et al. Carotid intima media thickness as a biomarker of subclinical atherosclerosis. Swiss Med Wkly. 2012;142:w13705. [DOI] [PubMed] [Google Scholar]
- 4. Desai CS, Ning H, Kang J, et al. Competing cardiovascular outcomes associated with subclinical atherosclerosis (from the Multi‐Ethnic Study of Atherosclerosis). Am J Cardiol. 2013;111:1541–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hassan A, Nazir SA, Alkadhi H. Technical challenges of coronary CT angiography: today and tomorrow. Eur J Radiol. 2011;79:161–171. [DOI] [PubMed] [Google Scholar]
- 6. Soon K, Wong C. Coronary computed tomography angiography: a new wave of cardiac imaging. Intern Med J . 2012;42(suppl 5):22–29. [DOI] [PubMed] [Google Scholar]
- 7. Min JK, Shaw LJ, Devereux RB, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all‐cause mortality. J Am Coll Cardiol. 2007;50:1161–1170. [DOI] [PubMed] [Google Scholar]
- 8. Zhang KY, Gai LY, Gai JJ, et al. Four‐year clinical outcome in asymptomatic patients undergoing coronary computed tomography angiography. Chin Med J (Engl). 2013;126:1630–1635. [PubMed] [Google Scholar]
- 9. Mason JE, Starke RD, Van Kirk JE. Gamma‐glutamyl transferase: a novel cardiovascular risk biomarker. Prev Cardiol. 2010;13:36–41. [DOI] [PubMed] [Google Scholar]
- 10. Turgut O, Tandogan I, Gurlek A. Association of gamma‐glutamyltransferase with cardiovascular risk: a prognostic outlook. Arch Med Res. 2009;40:318–320. [DOI] [PubMed] [Google Scholar]
- 11. Emdin M, Passino C, Michelassi C, et al. Additive prognostic value of gamma‐glutamyltransferase in coronary artery disease. Int J Cardiol. 2009;136:80–85. [DOI] [PubMed] [Google Scholar]
- 12. Lee DH, Jacobs DR Jr, Gross M, et al. Gamma‐glutamyltransferase is a predictor of incident diabetes and hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Clin Chem. 2003;49:1358–1366. [DOI] [PubMed] [Google Scholar]
- 13. Volpe M, Tocci G. 2007 ESH/ESC guidelines for the management of hypertension, from theory to practice: global cardiovascular risk concept. J Hypertens Suppl. 2009;27:S3–S11. [DOI] [PubMed] [Google Scholar]
- 14. Introduction: the American Diabetes Association's (ADA) evidence‐based practice guidelines, standards, and related recommendations and documents for diabetes care. Diabetes Care . 2012;35(suppl 1):S1–S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perry IJ, Wannamethee SG, Shaper AG. Prospective study of serum gamma‐glutamyltransferase and risk of NIDDM. Diabetes Care. 1998;21:732–737. [DOI] [PubMed] [Google Scholar]
- 16. European Association for Cardiovascular Prevention and Rehabilitation , Reiner Z, Catapano AL, De Backer G, et al. ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J . 2011;32:1769–1818. [DOI] [PubMed] [Google Scholar]
- 17. Austen WG, Edwards JE, Frye RL, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation . 1975;51(4 suppl):5–40. [DOI] [PubMed] [Google Scholar]
- 18. Ruttmann E, Brant LJ, Concin H, et al. Gamma‐glutamyltransferase as a risk factor for cardiovascular disease mortality: an epidemiological investigation in a cohort of 163,944 Austrian adults. Circulation. 2005;112:2130–2137. [DOI] [PubMed] [Google Scholar]
- 19. Wannamethee G, Ebrahim S, Shaper AG. γ‐Glutamyltransferase: determinants and association with mortality from ischemic heart disease and all causes. Am J Epidemiol . 1995;142:699–708. [DOI] [PubMed] [Google Scholar]
- 20. Ulus T, Yildirir A, Sade LE, et al. Serum gamma‐glutamyl transferase activity: new high‐risk criteria in acute coronary syndrome patients? Coron Artery Dis. 2008;19:489–495. [DOI] [PubMed] [Google Scholar]
- 21. Akpek M, Elcik D, Kalay N, et al. The prognostic value of serum gamma glutamyl transferase activity on admission in patients with STEMI undergoing primary PCI. Angiology. 2012;63:579–585. [DOI] [PubMed] [Google Scholar]
- 22. Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263–355. [DOI] [PubMed] [Google Scholar]
- 23. Paolicchi A, Emdin M, Ghliozeni E, et al. Images in cardiovascular medicine. Human atherosclerotic plaques contain gamma‐glutamyl transpeptidase enzyme activity. Circulation. 2004;109:1440. [DOI] [PubMed] [Google Scholar]
- 24. Lim JS, Yang JH, Chun BY, et al. Is serum gamma‐glutamyltransferase inversely associated with serum antioxidants as a marker of oxidative stress? Free Radic Biol Med. 2004;37:1018–1023. [DOI] [PubMed] [Google Scholar]
- 25. Paolicchi A, Minotti G, Tonarelli P, et al. Gamma‐glutamyl transpeptidase‐dependent iron reduction and LDL oxidation—a potential mechanism in atherosclerosis. J Invest Med. 1999;47:151–160. [PubMed] [Google Scholar]
- 26. Turgut O, Tandogan I. Gamma‐glutamyltransferase to determine cardiovascular risk: shifting the paradigm forward. J Atheroscler Thromb. 2011;18:177–1781. [DOI] [PubMed] [Google Scholar]
- 27. Cayli M, Gur M, Kalkan GY, et al. Gamma glutamyl transferase activity: Relationship with thoracic aortic intima media thickness and inflammation [published online ahead of print August 11, 2013]. Herz . doi: 10.1007/s00059-013-3921-0. [DOI] [PubMed] [Google Scholar]
- 28. Atar AI, Yilmaz OC, Akin K, et al. Association between gammaglutamyltransferase and coronary artery calcification. Int J Cardiol. 2013;167:1264–1267. [DOI] [PubMed] [Google Scholar]
- 29. Lee W, Ryoo JH, Suh BS, et al. Association of coronary artery calcification and serum gamma‐glutamyl transferase in Korean. Atherosclerosis. 2013;226:269–274. [DOI] [PubMed] [Google Scholar]
- 30. Bian LQ, Zhang ZY, Kim SJ, et al. Gamma glutamyltransferase as a novel marker of coronary artery calcification in women. J Cardiovasc Med (Hagerstown). 2012;13:684–690. [DOI] [PubMed] [Google Scholar]
- 31. Rubinshtein R, Gaspar T, Halon DA, et al. Prevalence and extent of obstructive coronary artery disease in patients with zero or low calcium score undergoing 64‐slice cardiac multidetector computed tomography for evaluation of a chest pain syndrome. Am J Cardiol. 2007;99:472–475. [DOI] [PubMed] [Google Scholar]
- 32. Cheng VY, Lepor NE, Madyoon H, et al. Presence and severity of noncalcified coronary plaque on 64‐slice computed tomographic coronary angiography in patients with zero and low coronary artery calcium. Am J Cardiol. 2007;99:1183–1186. [DOI] [PubMed] [Google Scholar]
- 33. Aggarwal NR, Knickelbine T, Tande A, et al. Noncalcified plaque: relationship between results of multislice computed tomography, risk factors, and late clinical outcome. Catheter Cardiovasc Interv. 2011;78:1116–1124. [DOI] [PubMed] [Google Scholar]
- 34. de Carvalho MS, de Araujo Goncalves P, Garcia‐Garcia HM, et al. Prevalence and predictors of coronary artery disease in patients with a calcium score of zero. Int J Cardiovasc Imaging. 2013;29:1839–1846. [DOI] [PubMed] [Google Scholar]
- 35. Jin KN, Chun EJ, Lee CH, et al. Subclinical coronary atherosclerosis in young adults: prevalence, characteristics, predictors with coronary computed tomography angiography. Int J Cardiovasc Imaging . 2012;28(suppl 2):93–100. [DOI] [PubMed] [Google Scholar]
- 36. Yoo DH, Chun EJ, Choi SI, et al. Significance of noncalcified coronary plaque in asymptomatic subjects with low coronary artery calcium score: assessment with coronary computed tomography angiography. Int J Cardiovasc Imaging . 2011;27(suppl 1):27–35. [DOI] [PubMed] [Google Scholar]


