ABSTRACT
Background
Past research has identified aortic stenosis (AS) as a major risk factor for adverse outcomes in noncardiac surgery; however, more contemporary studies have questioned the grave prognosis. To further our understanding of this, the risks of a 30‐day major adverse cardiovascular event (MACE) and all‐cause mortality were investigated in a contemporary Danish cohort.
Hypothesis
AS is not an independent risk factor for adverse outcomes in noncardiac surgery.
Methods
All patients with and without diagnosed AS who underwent noncardiac surgery in 2005 to 2011 were identified through nationwide administrative registers. AS patients (n = 2823; mean age, 75.5 years, 53% female) were matched with patients without AS (n = 2823) on propensity score for AS and surgery type.
Results
In elective surgery, MACE (ie, nonfatal myocardial infarction, ischemic stroke, or cardiovascular death) occurred in 66/1772 (3.7%) of patients with AS and 52/1772 (2.9%) of controls (P = 0.19), whereas mortality occurred in 67/1772 (3.8%) AS patients and 51/1772 (2.9%) controls (P = 0.13). In emergency surgery, 163/1051 (15.5%) AS patients and 120/1051 (11.4%) controls had a MACE (P = 0.006), whereas 225/1051 (21.4%) vs 179/1051 (17.0%) AS patients and controls died, respectively (P = 0.01). Event rates were higher for those with symptoms (defined as use of nitrates, congestive heart failure, or use of loop diuretics), compared with those without symptoms (P < 0.0001).
Conclusions
AS is associated with high perioperative rates of MACE and mortality, but perhaps prognosis is, in practice, not much worse for patients with AS than for matched controls. Symptomatic patients and patients undergoing emergency surgery are at considerable risks of a MACE and mortality.
Introduction
Aortic stenosis (AS) is an established risk factor for poor outcomes in noncardiac surgery.1, 2, 3 Much of the available data on clinical outcomes of AS has, however, been obtained from studies carried out more than a decade ago.3, 4 In recent times, surgical and anesthesia techniques, as well as cardiovascular medicine, have undergone significant changes, which may or may not have influenced the outcomes associated with AS in noncardiac surgery. A recent observational case‐control study of 256 patients with severe AS and 256 matched controls undergoing intermediate‐ or high‐risk noncardiac surgery (2000–2010) at the Mayo Clinic showed encouraging outcomes on 30‐day perioperative risk of mortality and major adverse cardiovascular event (MACE). Albeit increased, MACE rates were lower in the latter cohort than those of some of previous reports (18.8% in AS vs 10.5% in controls).5 Based on the study, Osnabrugge et al discussed in an accompanying editorial whether it was time to change guidelines and perhaps downplay the role of AS as a perioperative risk factor in noncardiac surgery.6 We undertook the present survey, based on Danish administrative nationwide registries, to further improve our understanding of the prognosis associated with AS in contemporary materials.
Methods
Registries and Study Population
Statistics Denmark holds several nationwide administrative healthcare‐related registries, which are accessible to researchers in a deidentified manner. The registries are complete and accurate because of the tax‐financed nationwide healthcare system, in which economical reimbursement to hospital departments is dependent on correct coding.7 We used 5 of these registries for the present survey. All of the registries have previously been used in research.8, 9 In brief, we used the Danish National Patient Registry to identify all patients with AS age ≥20 years who underwent noncardiac surgery between 2005 and 2011. Those with prior replacement surgery or percutaneous valve repair on the aortic valve (Nordic classification system code KFM) were not included. The diagnosis of AS was based on at least 1 outpatient or inpatient discharge code (International Classification of Diseases [ICD]‐10 codes I35.0 or I35.2) between 1994 and the surgery date. This definition has been used in previous Swedish and Danish samples, and has been validated with high specificity in the Swedish sample.10 The majority of patients with an AS diagnosis were reported to have moderate to severe AS in the previous survey.10 We considered patients to be symptomatic if they used nitrates or loop diuretics during the 120 days prior to surgery or had diagnosed heart failure. For comorbidities, we considered discharge diagnoses from any hospitalization up to 5 years prior to surgery date. The majority of diagnoses have been validated, all of which have good‐to‐excellent positive predictive values.11 The Danish National Patient registry was also used to identify all noncardiac, nonobstetric surgeries and included those who were also registered in the Danish anesthesia registry. From the Danish anesthesia registry we obtained information on body mass index (BMI), current smoking status, and whether the surgery was emergent or elective. We created 17 surgery subgroups according to surgical specialty and extent of surgery: “thoracic” surgery included pulmonary, mediastinal, and pleural surgery; “abdominal (bowel)” surgery included esophageal, gastric, duodenal, small intestine, colon, and rectal surgery; “abdominal (nonbowel)” included all other kinds of abdominal surgeries; “urology” included surgery of kidneys, ureters, and bladder; “male reproductive” included surgery to penis, urethra, scrotum, and prostate/seminal glands; “orthopedic minor” included hand, antebrachial, and foot surgery; “orthopedic major” included all other orthopedic surgeries; and “vascular (arteries)” included the whole arterial system, in accordance with prior work.8 Surgeries involving more than 1 surgical specialty were not included in our study. This approach was based on clinical judgment and the classification system developed by Poldermans et al.12
By employing the Danish Registry of Medicinal Product Statistics, use of selected cardiovascular drugs was identified through prescription claims any time up to 120 days prior to surgery. Because we had no data on heart failure severity, we used average daily used dosage of loop diuretics as a proxy when matching AS patients and controls.13, 14
Outcomes
We investigated the 30‐day risks of MACE (nonfatal ischemic stroke [ICD‐10 codes I63, I64], nonfatal acute myocardial infarction [AMI] [ICD‐10 code I21], or cardiovascular death [ICD‐10 codes I00–I99]), and all‐cause mortality. Vital status was obtained from the National Population Registry, which holds information on dates of birth and death for all individuals, and causes of death were obtained from the National Death Registry, which is based on information on death certificates. For nonfatal events, we only considered in‐hospital diagnoses, as these have been validated with good‐to‐excellent positive predictive values.15, 16
Ethics
The Danish Data Protection Agency approved this study (reference no. 2007‐58‐0015 int. ref: GEH‐2014‐019, 02737). In Denmark, retrospective registry‐based studies do not require approval from the ethics committees.
Statistics
We treated all surgeries as independent observations. For patients who had multiple surgeries performed in the same 30‐day period, we only included the first surgery. We calculated the propensity of having AS by logistic regression models including the following variables: sex, age, chronic obstructive pulmonary disease, diabetes, a history of anemia/bleeding, prior MI, kidney disease, rheumatic disease, peripheral artery disease, cerebrovascular disease, atrial fibrillation, heart failure, diabetes, current smoking, BMI category (underweight, normal, overweight, or obese), calendar year, and use of aspirin, clopidogrel, vitamin K antagonists, renin‐angiotensin system inhibitors, lipid modifying therapy, aldosterone blockers, and loop diuretic groups (furosemide equivalents of 0 mg/day, ≤40 mg/day, 41–80 mg/day, 81–160 mg/day, or >160 mg/day, respectively). The C statistic of the model was 0.886. We matched AS patients with controls in a 1:1 fashion (to maintain a high accuracy of matching) on propensity score, emergency vs elective surgery, and the 17 subgroups of surgery using the Greedy matching macro (www.mayo.edu/research/documents/gmatch.sas/DOC‐10027248). Difference in baseline characteristics and outcomes was tested by the χ2 test for discrete variables and the t test for continuous variables. Adjusted odds ratios were calculated by multivariable logistic regression models. All variables from Table 1 were included in the model (except for heart failure and loop diuretics, which were already included in the symptomatic variable), irrespectiveof significance level (a priori decided). We tested for interactions between aortic stenosis and emergency status by inclusion of an AS × emergency product term in the model. All analyses were performed in SAS version 9.4 (SAS Institute, Cary, NC). A 2‐sided P value <0.05 was considered significant for all tests.
Table 1.
Baseline Characteristics of AS Patients and Controls
| AS Patients | Controls | P for Difference | |
|---|---|---|---|
| Gender, female | 1487 (53%) | 1492 (53%) | 0.89 |
| Age, y | 75.5 (12.5) | 76.5 (12.2) | 0.002 |
| Body mass index group | 0.75 | ||
| Underweight (<18.5 kg/m2) | 155 (5.5%) | 151 (5.4%) | |
| Normal weight (18.5–24.9 kg/m2) | 1335 (47%) | 1361 (48%) | |
| Overweight (25.0–29.9 kg/m2) | 871 (31%) | 877 (31%) | |
| Obese (≥30 kg/m2) | 462 (16%) | 434 (15%) | |
| Current smoking | 405 (14%) | 412 (15%) | 0.79 |
| Symptomatica | 1206 (43%) | 1080 (38%) | 0.0006 |
| Comorbidities | |||
| Acute myocardial infarction | 225 (8.0%) | 225 (8.0%) | >0.99 |
| Prior CABG | 161 (5.7%) | 123 (4.4%) | 0.02 |
| Prior PCI | 246 (8.7%) | 221 (7.8%) | 0.23 |
| COPD | 267 (9.7%) | 274 (9.5%) | 0.75 |
| Anemia/bleeding disorder | 401 (14%) | 382 (14%) | 0.46 |
| Kidney disease | 197 (7.0%) | 177 (6.3%) | 0.28 |
| Peripheral artery disease | 281 (10%) | 269 (9.5%) | 0.59 |
| Cerebrovascular disease | 332 (12%) | 337 (12%) | 0.84 |
| Heart failure | 550 (20%) | 477 (17%) | 0.01 |
| Atrial fibrillation | 575 (20%) | 530 (19%) | 0.13 |
| Diabetes | 361 (13%) | 375 (13%) | 0.58 |
| Medications | |||
| Insulin | 153 (5.4%) | 156 (5.5%) | 0.86 |
| Clopidogrel | 150 (5.3%) | 164 (5.8%) | 0.42 |
| Vitamin K antagonists | 464 (16%) | 382 (14%) | 0.002 |
| Aspirin | 1274 (45%) | 1323 (47%) | 0.19 |
| Lipid modifying therapy | 1157 (41%) | 948 (34%) | <0.0001 |
| Beta blockers | 986 (35%) | 918 (33%) | 0.06 |
| Calcium blockers | 812 (29%) | 637 (23%) | <0.0001 |
| Loop diuretics | 914 (32%) | 827 (29%) | 0.01 |
| RAS inhibitors | 1126 (40%) | 1143 (40%) | 0.64 |
| Aldosterone blockers | 228 (8.1%) | 181 (6.4%) | 0.02 |
| Surgery characteristics | |||
| Emergency surgery | 1051 (37%) | 1051 (37%) | >0.99 |
| Cancer related surgery | 240 (8.5%) | 246 (8.7%) | 0.78 |
| Surgery subtype | >0.99 | ||
| Ear, nose, throat | 30 (1.1%) | 30 (1.1%) | |
| Orthopedic, major | 1154 (41%) | 1154 (41%) | |
| Orthopedic, minor | 224 (7.9%) | 224 (7.9%) | |
| Abdominal, nonbowel | 314 (11%) | 314 (11%) | |
| Abdominal, bowel | 207 (7.3%) | 207 (7.3%) | |
| Breast | 34 (1.2%) | 34 (1.2%) | |
| Plastic | 118 (4.2%) | 118 (4.2%) | |
| Endocrine | 18 (0.6%) | 18 (0.6%) | |
| Eye | 28 (1.0%) | 28 (1.0%) | |
| Female reproductive | 83 (2.9%) | 83 (2.9%) | |
| Intracranial | 43 (1.5%) | 43 (1.5%) | |
| Male reproductive | 14 (0.5%) | 14 (0.5%) | |
| Neuro | 66 (2.3%) | 66 (2.3%) | |
| Nonarterial vessels | 55 (2.0%) | 55 (2.0%) | |
| Pulmonary/mediastinal | 60 (2.1%) | 60 (2.1%) | |
| Urological | 211 (7.5%) | 211 (7.5%) | |
| Artery surgery | 164 (5.8%) | 164 (5.8%) |
Abbreviations: AS, aortic stenosis; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; PCI, percutaneous coronary intervention; RAS, renin‐angiotensin system.
Symptomatic is defined as use of nitrates, loop diuretics, or diagnosed heart failure.
Results
A total of 2823 patients with AS (mean age, 75.5 years; 53% women) were matched with 2823 controls. Baseline characteristics of AS patients and controls are presented in Table 1. As seen, AS patients and controls were very comparable on most variables, including the surgery‐related characteristics. Nearly half of all surgeries were of orthopedic subtype, with 41% being classified as major orthopedic and 8% as minor orthopedic surgical procedures. In total, 37% of surgeries were of emergency type in both groups. The presence of comorbidities was overall also similar between the AS patients and controls, with 8% having a history of myocardial infarction, 13% having diabetes, and 12% having cerebrovascular disease in both groups. The prevalence of atrial fibrillation was also not different between the groups—20% vs 19%—whereas diagnosed heart failure was more common among patients with AS (20% vs 17%, P = 0.01) compared with controls. The use of warfarin (16% vs 14%, P = 0.002) and lipid modifying therapy (41% vs 34%, P < 0.0001) was more frequent among AS patients compared with controls. AS patients were furthermore slightly younger than controls, with mean ages being 75.5 ± standard deviation 12.2 years vs 76.5 ± 12.5 years in AS patients and controls, respectively (P = 0.002). AS patents were significantly more often classified as symptomatic compared with controls (43% vs 38%, P = 0.0006).
Thirty‐Day Outcomes of MACE and Mortality
There was a significant difference in event rates for emergency and elective surgery in patients with AS and controls (P < 0.0001 for all comparisons, Table 2). Event rates were also significantly higher among those with symptoms, compared with those without symptoms for both AS patients and controls (P < 0.0001). Event rates for AS patients and controls, with and without symptoms, and undergoing emergency or elective surgery are presented in Figure 1.
Table 2.
Rates of 30‐Day MACE and Mortality in AS Patients and Controls Stratified by Emergency vs Elective Surgery
| AS Patients | Controls | P for Difference | |
|---|---|---|---|
| MACE | |||
| Emergency surgery | 163/1051 (15.5%) | 120/1051 (11.4%) | 0.006 |
| Elective surgery | 66/1772 (3.7%) | 52/1772 (2.9%) | 0.19 |
| Mortality | |||
| Emergency surgery | 225/1051 (21.4%) | 179/1051 (17.0%) | 0.01 |
| Elective surgery | 67/1772 (3.8%) | 51/1772 (2.9%) | 0.13 |
Abbreviations: AS, aortic stenosis; MACE, major adverse cardiovascular event.
Figure 1.
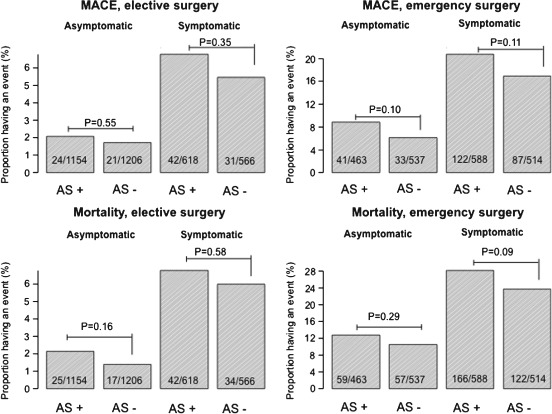
Event rates in different patient groups. Proportion of patients with aortic stenosis (AS+) and without aortic stenosis (AS−) experiencing a 30‐day event. Symptomatic refers to use of loop diuretics, nitrates, or diagnosed heart failure, and asymptomatic refers to all other patients. Abbreviations: MACE, major adverse cardiovascular event.
In multivariable logistic regression models of MACE, adjusted odds ratios were 1.58 (95% confidence interval [CI]: 1.08‐2.31) for AS patients without symptoms, 2.62 (95% CI: 1.84‐3.74) for AS patients with symptoms, and 1.91 (95% CI: 1.32‐2.76) for controls with symptoms compared with controls without symptoms. Similar results were shown for mortality; odds ratio 1.50 (95% CI: 1.07‐2.12) for AS patients without symptoms, 2.82 (95% CI: 1.52‐3.88) for AS patients with symptoms, and 2.12 (95% CI: 1.52‐2.96) for controls with symptoms compared with controls without symptoms. The odds ratio associated with AS was not significantly different for emergency vs elective surgery (P for interactions >0.4), although emergency surgery per se was associated with markedly increased risks (odds ratios 2.58 [95% CI: 2.00‐3.34] for MACE and 4.21 [95% CI: 3.30‐5.38] for mortality). Odds ratios for all other variables in models are presented in Figure 2.
Figure 2.
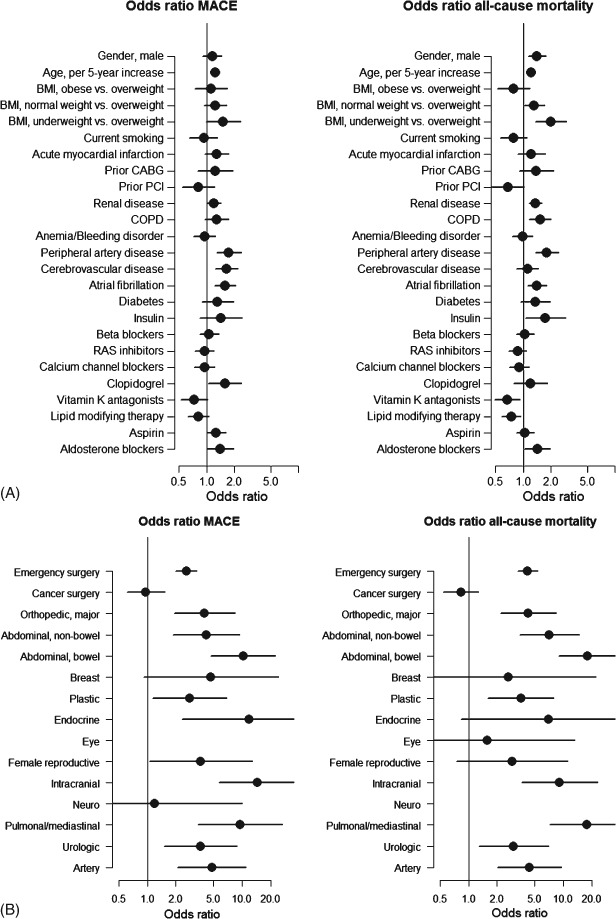
(A) Odds ratios for MACE and mortality associated with different variables. The odds ratios were obtained from multivariable analysis of cases and controls. (B) Odds ratios for MACE and mortality associated with different surgery‐related characteristics. The odds ratio estimates for different surgery subtypes (eg, abdominal, nonbowel) are compared with minor orthopedic surgery (Ref). Abbreviations: BMI, body mass index; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; MACE, major adverse cardiovascular event; PCI, percutaneous coronary intervention; RAS, renin‐angiotensin system.
Discussion
In the present study we investigated the 30‐day risks of MACE and mortality in a cohort of patients with AS and matched controls undergoing noncardiac surgery in Denmark between 2005 and 2011. In summary, we found that the event rates (ie, 30‐day risks of MACE and mortality) were moderate for elective surgeries (MACE 3.7% vs 2.9%; mortality 3.8% vs 2.9% in patients with and without AS), but very high for emergency surgery (mortality exceeded 20% for AS patients in emergency surgery).
Existing data on perioperative outcomes of noncardiac surgery in AS patients are sparse and conflicting overall, probably due to heterogeneous inclusion criteria and different control populations.17 In our study, we found that AS was associated with an odds ratio of 1.58 (95% CI: 1.08‐2.31) for MACE if asymptomatic and 2.82 (95% CI: 1.52‐3.88) if symptomatic compared with asymptomatic controls. It has previously been speculated that the reported poor prognosis associated with AS may be explained by great comorbidity burden and high‐risk surgery procedures rather than AS per se. As also demonstrated in the present study, AS patients had a significant burden of comorbidities including a high prevalence of prior myocardial infarction.18, 19 The matched control population had almost comparable high event rates, which may support the notion that comorbidities, rather than AS per se, are driving the high perioperative risks seen for AS patients undergoing noncardiac surgery. The adjusted odds ratio estimates for AS were, however, significant and comparable to those of kidney disease or peripheral artery disease, which underpin that AS needs to be regarded as an independent risk factor for adverse cardiovascular outcomes in noncardiac surgery, especially if symptomatic.
Similar to our study, a prior large observational study (n = 5149 patients with AS and 10284 controls) based on data from the US National Hospital Discharge Survey (1996–2002) reported that AS was associated with an adjusted odds ratio of 1.55 (95% CI: 1.26‐1.89) for perioperative mortality or acute myocardial infarction in noncardiac surgery (overall rates were 8.3% vs 7.2% among AS patients and controls).20 Somewhat higher event rates were reported in a Dutch survey of 108 patients with moderate or severe AS and 216 controls undergoing noncardiac surgery (38% underwent major vascular, 12% abdominal, and 21% orthopedic surgeries) in 1991 to 2000.2 Those with AS were reported to have significantly higher risks of mortality and nonfatal myocardial infarction, compared with controls in that study, despite being matched on type of surgery (14% vs 2%; adjusted odds ratio 5.2 [95% CI: 1.6‐17.0]).2 Using data from the Cleveland Clinic, Agarwal et al observed higher event rates (defined as mortality or myocardial infarction) in AS patients compared with matched controls undergoing elective noncardiac surgery (5.7% vs 2.7%, P = 0.02).21 Suggesting relatively good outcomes, a very recent study by Tashiro et al of patients with severe AS (as defined by echocardiography) and matched controls reported a mortality rate of 5.9% for those with AS and 3.1% for controls (P = 0.15).5 The surgery types were comparable to those in our study, but <10% of all surgeries were of the emergency type in the study by Tashiro et al, which likely have contributed to the much lower mortality rates.5 Interestingly, as also demonstrated in our study, the mortality rates were significantly higher in the symptomatic group (9.4%) compared with the asymptomatic group (3.3%) (P = 0.04) in the study by Tashiro et al.5
Strengths and Limitations
The major strength of the present study was the rather larger sample of patients with AS and matched controls with complete follow‐up. The main limitation was that it lacked data on echocardiography and clinical characteristics (such as chest x‐ray, electrocardiograms, and the presence of crackles), which could have helped refine risk stratification further, particularly so for AS patients. In our study we did not have information on the severity of AS, but patients most likely had moderate or severe AS (this was found in a comparable Swedish sample of patients with an ICD‐10 code of I35 [the Swedish healthcare system is very similar to the Danish system]).10 Our study was also limited by lack of blood biochemistry profiles and information on perioperative handling (eg, the use of Swan‐Ganz catheter/degree of monitoring as well as the use of perioperative medications and postoperative care).
Conclusion
AS was associated with high perioperative rates of MACE and mortality, but perhaps the prognosis is, in practice, not much worse for patients with AS than for matched controls. Given the high absolute adverse event rates, however, patients with AS may warrant special attention in a noncardiac surgery setting, in particular if symptomatic or undergoing emergency surgery. Our data furthermore support the current American and European guidelines, which acknowledge that AS is a risk factor for adverse outcomes in noncardiac surgery and recommend that noncardiac surgery should be postponed until after aortic valve replacement in case patients are symptomatic.12, 22
The study was founded by an independent research grant from the Danish Agency For Science, Technology and Innovation (grant no. FSS‐11‐120873). Dr. Gislason was supported by an independent research scholarship from the Novo Nordisk Foundation. The sponsors had no influence on design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Dr. Andersson has received a travel grant from AstraZeneca. Dr. Gislason has received speaker fees from AstraZeneca but are unrelated to the topic of the current study. Dr. Jensen has been a medical advisor in anesthesia for GlaxoSmithKline from 2008 to 2010 in Denmark.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Fleisher LA, Beckman JA, Brown KA, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120:e169–e276. [DOI] [PubMed] [Google Scholar]
- 2. Kertai MD, Bountioukos M, Boersma E, et al. Aortic stenosis: an underestimated risk factor for perioperative complications in patients undergoing noncardiac surgery. Am J Med. 2004;116:8–13. [DOI] [PubMed] [Google Scholar]
- 3. Detsky AS, Abrams HB, Forbath N, et al. Cardiac assessment for patients undergoing noncardiac surgery. A multifactorial clinical risk index. Arch Intern Med. 1986;146:2131–2134. [PubMed] [Google Scholar]
- 4. Goldman L, Caldera DL, Nussbaum SR, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297:845–850. [DOI] [PubMed] [Google Scholar]
- 5. Tashiro T, Pislaru SV, Blustin JM, et al. Perioperative risk of major non‐cardiac surgery in patients with severe aortic stenosis: a reappraisal in contemporary practice [published online ahead of print February 19, 2014]. Eur Heart J. doi: 10.1093/eurheartj/ehu044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Osnabrugge RL, Kappetein AP, Serruys PW. Non‐cardiac surgery in patients with severe aortic stenosis: time to revise the guidelines [published online ahead of print March 21, 2014]? Eur Heart J. doi: 10.1093/eurheartj/ehu116. [DOI] [PubMed] [Google Scholar]
- 7. Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39:30–33. [DOI] [PubMed] [Google Scholar]
- 8. Andersson C, Merie C, Jorgensen M, et al. Association of beta‐blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Intern Med. 2014;174:336–344. [DOI] [PubMed] [Google Scholar]
- 9. Jørgensen ME et al. Time elapsed after ischemic stroke and risk of adverse cardiovascular events and mortality following elective non cardiac surgery. JAMA. 2014;312(3):269–277. doi: 10.1001/jama.2014.8165. (PUBMED ID 25027142). [DOI] [PubMed] [Google Scholar]
- 10. Thanassoulis G, Campbell CY, Owens DS, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thygesen SK, Christiansen CF, Christensen S, et al. The predictive value of ICD‐10 diagnostic coding used to assess Charlson comorbidity index conditions in the population‐based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non‐cardiac Surgery; European Society of Cardiology (ESC) , Poldermans D, Bax JJ, Boersma E, et al. Guidelines for pre‐operative cardiac risk assessment and perioperative cardiac management in non‐cardiac surgery. Eur Heart J. 2009;30:2769–2812. [DOI] [PubMed] [Google Scholar]
- 13. Andersson C, Lyngbaek S, Nguyen CD, et al. Association of clopidogrel treatment with risk of mortality and cardiovascular events following myocardial infarction in patients with and without diabetes. JAMA. 2012;308:882–889. [DOI] [PubMed] [Google Scholar]
- 14. Andersson C, Norgaard ML, Hansen PR, et al. Heart failure severity, as determined by loop diuretic dosages, predicts the risk of developing diabetes after myocardial infarction: a nationwide cohort study. Eur J Heart Fail. 2010;12:1333–1338. [DOI] [PubMed] [Google Scholar]
- 15. Joensen AM, Jensen MK, Overvad K, et al. Predictive values of acute coronary syndrome discharge diagnoses differed in the Danish National Patient Registry. J Clin Epidemiol. 2009;62:188–194. [DOI] [PubMed] [Google Scholar]
- 16. Krarup LH, Boysen G, Janjua H, et al. Validity of stroke diagnoses in a National Register of Patients. Neuroepidemiology. 2007;28:150–154. [DOI] [PubMed] [Google Scholar]
- 17. Booher AM, Eagle KA. Noncardiac surgery in patients with aortic stenosis. http://www.uptodate.com/contents/noncardiac‐surgery‐in‐patients‐with‐aortic‐stenosis?source=search_result&search=aotic+stenosis&selectedTitle=13∼150. Accessed May 15, 2014.
- 18. Chobadi R, Wurzel M, Teplitsky I, et al. Coronary artery disease in patients 35 years of age or older with valvular aortic stenosis. Am J Cardiol. 1989;64:811–812. [DOI] [PubMed] [Google Scholar]
- 19. Faggiano P, Frattini S, Zilioli V, et al. Prevalence of comorbidities and associated cardiac diseases in patients with valve aortic stenosis. Potential implications for the decision‐making process. Int J Cardiol. 2012;159:94–99. [DOI] [PubMed] [Google Scholar]
- 20. Zahid M, Sonel AF, Saba S, et al. Perioperative risk of noncardiac surgery associated with aortic stenosis. Am J Cardiol. 2005;96:436–438. [DOI] [PubMed] [Google Scholar]
- 21. Agarwal S, Rajamanickam A, Bajaj NS, et al. Impact of aortic stenosis on postoperative outcomes after noncardiac surgeries. Circ Cardiovasc Qual Outcomes. 2013;6:193–200. [DOI] [PubMed] [Google Scholar]
- 22. Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116:e418–e499. [DOI] [PubMed] [Google Scholar]


