Abstract
Objective:
Patient-reported outcome scales determine response to treatment. The minimal clinically important difference (MCID) of these scales is a measure of responsiveness: the smallest change in a score associated with a clinically important change to the patient. This study sought to summarize the literature on MCID for the most commonly reported shoulder outcome scales.
Design:
A literature search of PubMed and EMBASE databases identified 193 citations, twenty-seven of which met the inclusion/exclusion criteria.
Results:
For rotator cuff tears, a MCID range of 9–26.9 was reported for American Shoulder and Elbow Surgeons (ASES), 8 or 10 for Constant, and 282.6–588.7 for the Western Ontario Rotator Cuff Index (WORC). For patients who underwent arthroplasty, a MCID range of 6.3–20.9 was reported for ASES, 5.7–9.4 for Constant, and 14.1–20.6 for the Shoulder Pain and Disability Index (SPADI). For proximal humeral fractures, a MCID range of 5.4–11.6 was reported for Constant and 8.1–13.0 for Disability of the Arm, Shoulder, and Hand (DASH).
Conclusion:
A wide range of MCID values was reported for each patient population and instrument. In the future, a uniform outcome instrument and MCID will be useful to measure clinically meaningful change across practices and the spectrum of shoulder diagnoses.
Keywords: Minimal clinically important difference, rotator cuff, shoulder disease, outcome assessment
Introduction
Patient-reported outcome scales are used to determine patients’ response to treatment. These instruments capture change in a patient’s clinical status over time, a property called responsiveness, which can be measured in multiple ways1. The minimal clinically important difference (MCID) is a measure of responsiveness defined as “the smallest difference in score in the domain of interest which patients perceive as beneficial and which would mandate, in the absence of troublesome side effects and excessive cost, a change in the patient’s management”2. In other words, the MCID is the smallest change in an outcome score that is associated with a clinically important change to the patient. This value helps avoid changes in score that may be statistically significant but do not result in meaningful change for the patient2.
Outcome instruments such as the Shoulder Pain and Disability Index (SPADI) and the American Shoulder and Elbow Surgeons Standardized Shoulder Form (ASES) are frequently used in populations with shoulder disorders including rotator cuff disease and proximal humeral fractures, and many studies have determined MCID values for standardized instruments3,4. However, there is variation in MCID values presented across studies that analyze the same outcome measures and patient populations1. The purpose of this study was to determine the ranges of MCID values in the current literature for different shoulder outcome measures and shoulder diagnoses.
Materials and Methods
A systematic literature search of PubMed and EMBASE databases was performed from their years of inception through March 2018. Keywords included “outcome scale,” “shoulder pain,” and “MCID.” The full search criteria can be found in Appendix A. A total of 193 citations were initially identified, and the titles and abstracts were reviewed for relevance. The full texts of 26 of these citations were then reviewed, and 9 were found to be relevant to the topic. Bibliographies of the full text articles were also reviewed, and an additional 18 papers were found to be relevant to the study. The inclusion criteria included studies that calculated the MCID of outcome measures for different shoulder diagnoses. Exclusion criteria were studies that analyzed non-English translated versions of an outcome instrument, did not calculate or explain how they calculated the MCID, or calculated a MCID value that included neck or distal upper extremity diagnoses (upper arm, elbow, forearm, wrist, or hand).
The Methodological Index for Non-Randomized Studies (MINORS) was used to assess the quality of the studies included in this review5. As these were non-comparative studies, the maximum possible score was 16. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology was used for reporting this manuscript (see Supplementary Checklist)6.
Results
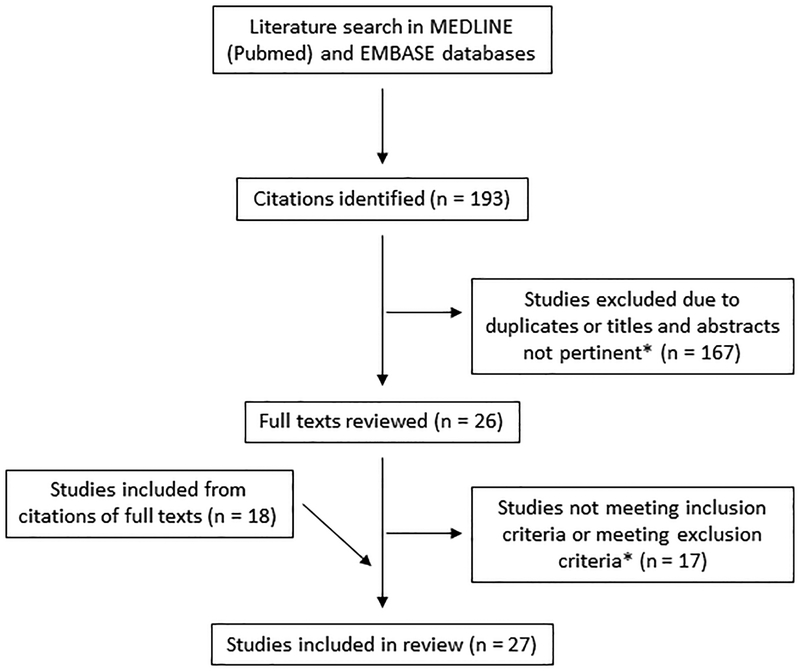
Twenty-seven studies that met the inclusion and exclusion criteria were included in the final analysis (Figure 1, Appendix B). Some of the most commonly-used outcome instruments represented in these articles were ASES, the Constant Shoulder Score, the Simple Shoulder Test (SST), and SPADI (Table I). Four general outcome measures were represented as well: the Visual Analog Scale (VAS) for pain, the Numerical Pain Rating Scale (NPRS), the Short Form-12 (SF-12), and the Numerical Function Rating Scale (NFRS). Twenty-one studies used an anchor-based method to calculate the MCID, thirteen used a distribution-based approach, and seven used a receiver operator curve (ROC) analysis. Many of these studies determined MCID values for different subgroups of their patient populations; however, only the overall results are included here to enable comparison.
Figure 1.
PRISMA diagram of the literature search and study selection
*Inclusion criteria: studies that calculated the MCID of outcome measures for different shoulder diagnoses. Exclusion criteria: studies that analyzed non-English translated versions of an outcome instrument, did not calculate or explain how they calculated the MCID, or calculated a MCID value that included neck or distal upper extremity diagnoses.
Table I.
The shoulder-specific questionnaires represented in the included studies, the interpretation of their scores, and the number of studies in which their MCID was calculated.
| Score Range | ||||
|---|---|---|---|---|
| Outcome Instrument | Abbreviation | # of studies | Worse shoulder function | Better shoulder function |
| American Shoulder and Elbow Surgeons Standardized Shoulder Form | ASES | 7 | 0 | 100 |
| Constant Shoulder Score | Constant | 4 | 0 | 100 |
| Simple Shoulder Test | SST | 4 | 0 | 12 |
| Shoulder Pain and Disability Index | SPADI | 4 | 100 | 0 |
| Disability of the Arm, Shoulder, and Hand | DASH | 3 | 100 | 0 |
| Shoulder Rating Questionnaire | SRQ | 3 | 17 | 100 |
| UCLA Shoulder Rating Scale | UCLA | 2 | 2 | 35 |
| Western Ontario Rotator Cuff Index | WORC | 2 | 2100 | 0 |
| Western Ontario Shoulder Instability Index | WOSI | 2 | 2100 | 0 |
| Shortened DASH | QuickDASH | 1 | 100 | 0 |
| Penn Shoulder Score | PSS | 1 | 0 | 100 |
| Rowe Score for Instability | Rowe | 1 | 0 | 100 |
| Oxford Instability Shoulder Score | OISS | 1 | 0 | 48 |
| Oxford Shoulder Score | OSS | 1 | 0 | 48 |
| Subjective Shoulder Value | SSV | 1 | 0 | 100 |
| Functional Shoulder Score | FSS | 1 | 0 | 100 |
| Flexilevel Scale of Shoulder Function | FLEX-SF | 1 | 0 | 100 |
| Shoulder Function Index | SFInX | 1 | 0 | 100 |
| Dutch Shoulder Disability Questionnaire | SDQ-NL | 1 | 100 | 0 |
| United Kingdom Shoulder Disability Questionnaire | SDQ-UK | 1 | 100 | 0 |
Ten studies either did not specify the shoulder disorders of their subjects or calculated a single MCID for multiple disorders (Table II). Of these ten, one calculated a MCID for ASES of 6.47. The Disability of the Arm, Shoulder, and Hand (DASH) had a MCID range of 3.9–158,9. MCID values of 1.1 and 2.17 were calculated for NPRS10,11. SPADI had MCID values of 8, 10, and 13.24,9,12.
Table II.
MCID values for unspecified shoulder disorder, rotator cuff disease, and rotator cuff tears specifically
| Outcome Instrument | MCID | References |
|---|---|---|
| Unspecified Shoulder Disorder | ||
| ASES | 6.4 | Michener et al.7 (2002) |
| DASH | 3.9–15 | Beaton et al.8 (2011) Schmitt et al.9 (2004) |
| FLEX-SF | 3.02 | Cook et al.30 (2003) |
| NPRS | 1.1, 2.17 | Michener et al.10 (2011) Mintken et al.11 (2009) |
| PSS | 11.4 | Leggin et al.1 (2006) |
| QuickDASH | 8 | Mintken et al.11 (2009) |
| SDQ-NL | 14 | Paul et al.4 (2004) |
| SDQ-UK | 4–8 | Paul et al.4 (2004) |
| SF-12 PCS | 6.5 | Schmitt et al.9 (2004) |
| SPADI | 8, 10, 13.2 | Paul et al.4 (2004) Schmitt et al.9 (2004) Williams et al.12 (1995) |
| SRQ | 12, 13 | L’Insalata et al.29 (1997) Paul et al.4 (2004) |
| Rotator Cuff Disease | ||
| ASES | 6.2–17, 17.9, 21.9, 26.9 | Gagnier et al.3 (2018) Tashjian et al.13 (2010) Werner et al.14 (2016) |
| Constant | 8, 10 | Kukkonen et al.15 (2013) Torrens et al.16 (2016) |
| FSS | 24.7 | Iossifidis et al.17 (2015) |
| NFRS | 15.1–37.3 | Tubach et al.25 (2006)* |
| NPRS | 34.3–62.5 | Tubach et al.25 (2006)* |
| Pain VAS | 1.37 | Tashjian et al.26 (2009) |
| SST | 2 | Tashjian et al.13 (2010) |
| WORC | 245.26, 282.6, 392.5, 588.7 | Gagnier et al.3 (2018)* Kirkley et al.24 (2003) |
| Rotator Cuff Tears | ||
| ASES | 6.2–13.9, 17.9, 21.9, 26.9 | Gagnier et al.3 (2018) Werner et al.14 (2016) |
| Constant | 8, 10 | Kukkonen et al.15 (2013) Torrens et al.16 (2016) |
| WORC | 282.6, 392.5, 588.7 | Gagnier et al.3 (2018)* |
MCID initially reported as negative because lower numbers indicated improvement but switched here for consistency and ease of comparison
Nine studies calculated a MCID for rotator cuff disease with or without a tear (Table II). ASES was found to have MCIDs of 6.2–17, 17.9, 21.9, and 26.93,13,14; Constant of 8 and 1015,16; and the Functional Shoulder Score (FSS) of 24.717. Tubach et al. in 2006 calculated separate MCID values for a numerical rating scale based on function and another based on pain, resulting in ranges of 15.1–37.3 and 34.3–62.5, respectively. A MCID of 2 was calculated for SST13.
Four studies calculated a MCID specifically for patients with rotator cuff tears (Table II). MCIDs of 6.2–13.9, 17.9, 21.9, and 26.9 were calculated for ASES3,14; 8 and 10 for Constant15,16; and 282.6, 392.5, and 588.7 for the Western Ontario Rotator Cuff Index (WORC)3.
Six studies calculated a MCID value for patients who underwent shoulder arthroplasty (Table III). A MCID range of 6.3–13.6 and 20.9 was reported for ASES14,18,19,20 and 5.7, 8, and 9.4 for Constant score16,18. SPADI was found to have MCID values of 14.1 and 20.618,19 and SST of 1.5, 1.8, 2.4, and 3.018,19,21.
Table III.
MCID values for shoulder arthroplasty, shoulder instability, and proximal humeral fractures
| Outcome Instrument | MCID | References |
|---|---|---|
| Shoulder Arthroplasty | ||
| ASES | 6.3–13.6, 20.9 | Simovitch et al.18 (2018) Tashjian et al.19 (2017) Werner et al.14 (2016) Wong et al.20 (2016) |
| Constant | 5.7, 8, 9.4 | Simovitch et al.18 (2018) Torrens et al.16 (2016) |
| SF-12 MCS | 5.7 | Wong et al.20 (2016) |
| SF-12 PCS | 5.4 | Wong et al.20 (2016) |
| SPADI | 14.1, 20.6 | Simovitch et al.18 (2018) |
| SST | 1.5, 1.8, 2.4, 3.0 | Roy et al.21 (2010) Simovitch et al.18 (2018) Tashjian et al.19 (2017) |
| UCLA | 3.6, 8.7 | Simovitch et al.18 (2018) |
| VAS Pain | 1.4, 1.6 | Simovitch et al.18 (2018) Tashjian et al.19 (2017) |
| Shoulder Instability | ||
| OISS | 4.5, 6.5 | Moser et al.22 (2008) |
| Rowe | 2.2, 5.6, 9.7 | Park et al.23 (2018) |
| SRQ | 4, 5 | Moser et al.22 (2008) |
| WOSI | 60.7, 151.9, 220 | Kirkley et al.24 (2003) Park et al.23 (2018) |
| Proximal Humeral Fractures | ||
| Constant | 5.4, 11.6 | Van de Water et al.27 (2014) |
| DASH | 8.1, 13.0 | Van de Water et al.27 (2014)* |
| OSS | 5.1, 11.4 | Van de Water et al.27 (2014) |
| SFInX | 10.3, 11.7 | Van de Water et al.28 (2016) |
| SSV | 12.1, 26.6 | Van de Water et al.27 (2014) |
| UCLA | 2.0, 2.4 | Van de Water et al.27 (2014) |
MCID initially reported as negative because lower numbers indicated improvement but switched here for consistency and ease of comparison
Three studies calculated a MCID for patients with shoulder instability (Table III). The Oxford Instability Shoulder Score (OISS) was found to have MCIDs of 4.5 and 6.522 and the Rowe Score for Instability of 2.2, 5.6, and 9.723. MCIDs of 4 and 5 were calculated for the Shoulder Rating Questionnaire (SRQ)22; and 60.7, 151.9, and 220 for the Western Ontario Shoulder Instability Index (WOSI)23,24.
Two studies calculated a MCID for proximal humeral fractures (Table III). Van de Water et al. in 2014 found MCID values of 5.4 and 11.6 for Constant, 8.1 and 13.0 for DASH, 5.1 and 11.4 for the Oxford Shoulder Score (OSS), 12.1 and 26.6 for the Subjective Shoulder Value (SSV), and 2.0 and 2.4 for the UCLA Shoulder Rating Scale. In 2016, the same authors calculated MCIDs of 10.3 and 11.7 for the Shoulder Function Index (SFInX).
The results of the MINORS criteria bias assessment are in Table IV5. All of the studies had a clearly stated aim, prospective collection of data, end points appropriate to the aim of the study, and a follow-up period appropriate to the aim of the study. Twenty-two studies included consecutive patients3,4,7,8,9,10,11,12,13,14,15,16,17,18,19,20,22,23,25,26,27,28, six stated they had unbiased assessment of the study end point9,10,21,23,27,28, four had less than 5% loss to follow-up13,15,16,23, and four had prospective calculation of the study size3,25,27,28. The lowest score was 91,24,29,30, and the highest was 1527,28.
Table IV.
Methodological Index for Non-Randomized Studies (MINORS) scores
| A clearly stated aim | Inclusion of consecutive patients | Prospective collection of data | End points appropriate to the aim of the study | Unbiased assessment of the study end point | Follow-up period appropriate to the aim of the study | Loss to follow-up <5% | Prospective calculation of study size | Total | |
|---|---|---|---|---|---|---|---|---|---|
| Beaton et al.8 (2011) | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 10 |
| Cook et al.30 (2003) | 2 | 0 | 2 | 2 | 0 | 2 | 1 | 0 | 9 |
| Gagnier et al.3 (2018) | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 2 | 13 |
| Iossifidis et al.17 (2015) | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 10 |
| Kirkley et al.24 (2003) | 2 | 1 | 2 | 2 | 0 | 2 | 0 | 0 | 9 |
| Kukkonen et al.15 (2013) | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 |
| L’Insalata et al.29 (1997) | 2 | 0 | 2 | 2 | 0 | 2 | 1 | 0 | 9 |
| Leggin et al.1 (2006) | 2 | 0 | 2 | 2 | 0 | 2 | 1 | 0 | 9 |
| Michener et al.7 (2002) | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 0 | 11 |
| Michener et al.10 (2011) | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 12 |
| Mintken et al.11 (2009) | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 10 |
| Moser et al.22 (2008) | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 0 | 11 |
| Park et al.23 (2018) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 14 |
| Paul et al.4 (2004) | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 0 | 11 |
| Roy et al.21 (2010) | 2 | 1 | 2 | 2 | 2 | 2 | 1 | 0 | 12 |
| Schmitt et al.9 (2004) | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 0 | 13 |
| Simovitch et al.18 (2018) | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 0 | 11 |
| Tashjian et al.26 (2009) | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 1 | 11 |
| Tashjian et al.13 (2010) | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 |
| Tashjian et al.19 (2017) | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 0 | 11 |
| Torrens et al.16 (2016) | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 |
| Tubach et al.25 (2006) | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 2 | 13 |
| Van de Water et al.27 (2014) | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 15 |
| Van de Water et al.28 (2016) | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 15 |
| Werner et al.14 (2016) | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 0 | 11 |
| Williams et al.12 (1995) | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 0 | 11 |
| Wong et al.20 (2016) | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 0 | 11 |
Discussion
The MCID measures the responsiveness of a scale by capturing the smallest change representative of a clinically important difference to the patient2. This study is a systematic review of MCID values reported for commonly used outcome scales in shoulder disorders. The ASES, Constant, SST, and SPADI were the most commonly used instruments to assess MCID in patients with shoulder disorders. Patient populations with rotator cuff disease or tears, with proximal humeral fractures, and after shoulder arthroplasty were assessed using these outcome scales. A wide range of MCID was reported for these instruments for each of the patient populations.
Our results show that the MCID for the same outcome instrument varies with the shoulder diagnosis that was studied. For instance, the ASES score has a different MCID for rotator cuff tears versus shoulder arthroplasty. Hence, it is important that the specific shoulder diagnosis be considered when reporting the MCID in research and clinical settings. A given MCID may not be applicable to a very heterogenous shoulder patient population with varying diagnoses.
No method for calculating the MCID is considered the gold standard. Three methods are most commonly used: the anchor-based approach, receiver operator curve (ROC) analysis, and distribution-based approach. The anchor-based approach correlates the change in score of the outcome instrument with an external anchoring question that measures improvement2. These anchors include terms such as “a little better” or “slightly better” to assess improvement. A broader version of the term anchoring to changes on a 4-point scale or to terms such as a “satisfactory outcome,” “a good deal better,” “a great deal better,” or “much better” was also used by some studies. ROC curves are graphs of sensitivity versus 1-specificity, and the upper left-most point – as the most sensitive and specific – is considered the cut off that can best distinguish patients who have improved from those who have not7. Distribution-based methods use a statistical measurement such as the standard deviation or standard error of measurement of the sample8. A fourth approach is consensus-based, which involves surveying the opinions of experts to determine a reasonable value; however, this method is less frequently accepted.
In general, a relatively wide range of MCID values was observed for the outcome scales and shoulder diagnoses included in the study. This is likely because of the heterogeneity in patient populations recruited for the studies and variation in methodology used to calculate MCID. There is no identifiable pattern between the method used to calculate the MCID and the differences in results; however, none of the studies that examined rotator cuff disease, rotator cuff tears specifically, or proximal humeral fractures used an ROC curve.
This study had a couple limitations. This analysis had studies3,8,13,15,16,23,24,27,28,30 that included patients who improved and those who worsened in their anchor-based MCID calculations as well as studies1,9,14,17,18,22,25,29 that only included patients who improved, leading to variations in MCID scores across studies. In addition, many of these studies determined MCID values for different subgroups of their patient populations, but only the overall results were included here to enable comparison. Analysis of the subgroup MCID values may alter the results.
Conclusions
We present existing data on MCID for commonly used outcomes instruments for shoulder disorders and discuss some of the methodologies used in the studies. The ASES, Constant, SST, and SPADI were the most commonly used instruments to assess MCID in patients with shoulder disorders. Patient populations with rotator cuff disease or tears, with proximal humeral fractures, and after shoulder arthroplasty were assessed using these outcome scales. A wide range of MCID values was reported for these instruments for each shoulder patient population. Information from our study can be used by professional agencies to determine whether available evidence is sufficient to recommend uniform outcomes instrument(s) with acceptable MCID for various shoulder disorders.
Supplementary Material
Appendix A. Search criteria used in PubMed and EMBASE databases
Appendix B. Table consisting of a summary of the participants, outcome scales, inclusion and exclusion criteria, MCID calculation, and results of each paper included in this review
Preferred Reporting Items for Systematic Reviews and Meta-Analayses (PRISMA) Checklist
Source of Funding
Dr. Jain is/was supported by funding from NIAMS 1K23AR059199 and 1U34AR069201.
References
- 1.Leggin BG, Michener LA, Shaffer MA, Brenneman SK, Iannotti JP, Williams GR Jr. The Penn shoulder score: reliability and validity. J Orthop Sports Phys Ther 2006; 36(3):138–151. [DOI] [PubMed] [Google Scholar]
- 2.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 1989; 10(4):407–415. [DOI] [PubMed] [Google Scholar]
- 3.Gagnier JJ, Robbins C, Bedi A, Carpenter JE, Miller BS. Establishing minimally important differences for the American Shoulder and Elbow Surgeons score and the Western Ontario Rotator Cuff Index in patients with full-thickness rotator cuff tears. J Shoulder Elbow Surg 2018; 27(5):e160–e166. [DOI] [PubMed] [Google Scholar]
- 4.Paul A, Lewis M, Shadforth MF, Croft PR, Van Der Windt DA, Hay EM. A comparison of four shoulder-specific questionnaires in primary care. Ann Rheum Dis 2004; 63(10):1293–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003; 73(9):712–716. [DOI] [PubMed] [Google Scholar]
- 6.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg 2002; 11(6):587–594. [DOI] [PubMed] [Google Scholar]
- 8.Beaton DE, van Eerd D, Smith P, et al. Minimal change is sensitive, less specific to recovery: a diagnostic testing approach to interpretability. J Clin Epidemiol 2011; 64(5):487–496. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt JS, Di Fabio RP. Reliable change and minimum important difference (MID) proportions facilitated group responsiveness comparisons using individual threshold criteria. J Clin Epidemiol 2004; 57(10):1008–1018. [DOI] [PubMed] [Google Scholar]
- 10.Michener LA, Snyder AR, Leggin BG. Responsiveness of the numeric pain rating scale in patients with shoulder pain and the effect of surgical status. J Sport Rehabil 2011; 20(1):115–128. [DOI] [PubMed] [Google Scholar]
- 11.Mintken PE, Glynn P, Cleland JA. Psychometric properties of the shortened disabilities of the Arm, Shoulder, and Hand Questionnaire (QuickDASH) and Numeric Pain Rating Scale in patients with shoulder pain. J Shoulder Elbow Surg 2009; 18(6):920–926. [DOI] [PubMed] [Google Scholar]
- 12.Williams JW Jr., Holleman DR Jr., Simel DL. Measuring shoulder function with the Shoulder Pain and Disability Index. J Rheumatol 1995; 22(4):727–732. [PubMed] [Google Scholar]
- 13.Tashjian RZ, Deloach J, Green A, Porucznik CA, Powell AP. Minimal clinically important differences in ASES and simple shoulder test scores after nonoperative treatment of rotator cuff disease. J Bone Joint Surg Am 2010; 92(2):296–303. [DOI] [PubMed] [Google Scholar]
- 14.Werner BC, Chang B, Nguyen JT, Dines DM, Gulotta LV. What Change in American Shoulder and Elbow Surgeons Score Represents a Clinically Important Change After Shoulder Arthroplasty? Clin Orthop Relat Res 2016; 474(12):2672–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kukkonen J, Kauko T, Vahlberg T, Joukainen A, Aarimaa V. Investigating minimal clinically important difference for Constant score in patients undergoing rotator cuff surgery. J Shoulder Elbow Surg 2013; 22(12):1650–1655. [DOI] [PubMed] [Google Scholar]
- 16.Torrens C, Guirro P, Santana F. The minimal clinically important difference for function and strength in patients undergoing reverse shoulder arthroplasty. J Shoulder Elbow Surg 2016; 25(2):262–268. [DOI] [PubMed] [Google Scholar]
- 17.Iossifidis A, Ibrahim EF, Petrou C, Galanos A. The development and validation of a questionnaire for rotator cuff disorders: The Functional Shoulder Score. Shoulder Elbow 2015; 7(4):256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simovitch R, Flurin PH, Wright T, Zuckerman JD, Roche CP. Quantifying success after total shoulder arthroplasty: the minimal clinically important difference. J Shoulder Elbow Surg 2018; 27(2):298–305. [DOI] [PubMed] [Google Scholar]
- 19.Tashjian RZ, Hung M, Keener JD, et al. Determining the minimal clinically important difference for the American Shoulder and Elbow Surgeons score, Simple Shoulder Test, and visual analog scale (VAS) measuring pain after shoulder arthroplasty. J Shoulder Elbow Surg 2017; 26(1):144–148. [DOI] [PubMed] [Google Scholar]
- 20.Wong SE, Zhang AL, Berliner JL, Ma CB, Feeley BT. Preoperative patient-reported scores can predict postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg 2016; 25(6):913–919. [DOI] [PubMed] [Google Scholar]
- 21.Roy JS, Macdermid JC, Faber KJ, Drosdowech DS, Athwal GS. The simple shoulder test is responsive in assessing change following shoulder arthroplasty. J Orthop Sports Phys Ther 2010; 40(7):413–421. [DOI] [PubMed] [Google Scholar]
- 22.Moser JS, Barker KL, Doll HA, Carr AJ. Comparison of two patient-based outcome measures for shoulder instability after nonoperative treatment. J Shoulder Elbow Surg 2008; 17(6):886–892. [DOI] [PubMed] [Google Scholar]
- 23.Park I, Lee JH, Hyun HS, Lee TK, Shin SJ. Minimal clinically important differences in Rowe and Western Ontario Shoulder Instability Index scores after arthroscopic repair of anterior shoulder instability. J Shoulder Elbow Surg 2018; 27(4):579–584. [DOI] [PubMed] [Google Scholar]
- 24.Kirkley A, Griffin S, Dainty K. Scoring systems for the functional assessment of the shoulder. Arthroscopy 2003; 19(10):1109–1120. [DOI] [PubMed] [Google Scholar]
- 25.Tubach F, Dougados M, Falissard B, Baron G, Logeart I, Ravaud P. Feeling good rather than feeling better matters more to patients. Arthritis Rheum 2006; 55(4):526–530. [DOI] [PubMed] [Google Scholar]
- 26.Tashjian RZ, Deloach J, Porucznik CA, Powell AP. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease. J Shoulder Elbow Surg 2009; 18(6):927–932. [DOI] [PubMed] [Google Scholar]
- 27.van de Water AT, Shields N, Davidson M, Evans M, Taylor NF. Reliability and validity of shoulder function outcome measures in people with a proximal humeral fracture. Disabil Rehabil 2014; 36(13):1072–1079. [DOI] [PubMed] [Google Scholar]
- 28.van de Water AT, Davidson M, Shields N, Evans MC, Taylor NF. The Shoulder Function Index (SFInX): evaluation of its measurement properties in people recovering from a proximal humeral fracture. BMC Musculoskelet Disord 2016; 17:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.L’Insalata JC, Warren RF, Cohen SB, Altchek DW, Peterson MG. A self-administered questionnaire for assessment of symptoms and function of the shoulder. J Bone Joint Surg Am 1997; 79(5):738–748. [PubMed] [Google Scholar]
- 30.Cook KF, Roddey TS, Gartsman GM, Olson SL. Development and psychometric evaluation of the Flexilevel Scale of Shoulder Function. Med Care 2003; 41(7):823–835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A. Search criteria used in PubMed and EMBASE databases
Appendix B. Table consisting of a summary of the participants, outcome scales, inclusion and exclusion criteria, MCID calculation, and results of each paper included in this review
Preferred Reporting Items for Systematic Reviews and Meta-Analayses (PRISMA) Checklist