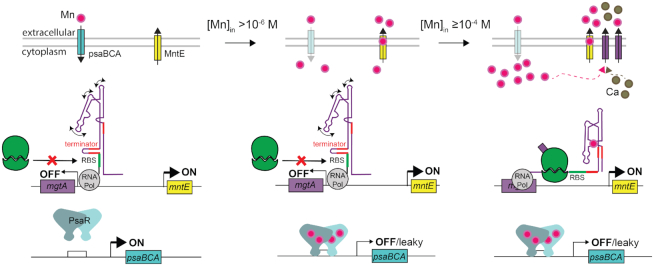
Figure 11.
Threshold model for Mn2+ sensing and detoxification in S. pneumoniae. The Mn2+ binding affinity of the S. pneumoniae mgtA riboswitch is such that there is sufficient free intracellular Mn2+, governed by the relative affinities of the transcriptional Mn uptake repressor PsaR and the constitutively expressed Mn-specific efflux pump MntE (34,57), to ensure MgtA is not induced and that key intracellular Mn-dependent enzymes are active. At concentrations >1 μM Mn2+, PsaR will bind Mn2+, increasing its affinity to DNA and repress psaBCA transcription, thereby reducing Mn import; excess Mn2+ will continue to be effluxed by MntE. Our data suggest that as free Mn2+ rises to ≥100 μM, the mgtA riboswitch functions as a failsafe ‘on’ signal inducing MgtA expression to prevent Mn2+ toxicity. In addition, the riboswitch also functions to regulate Ca2+ efflux under this condition.