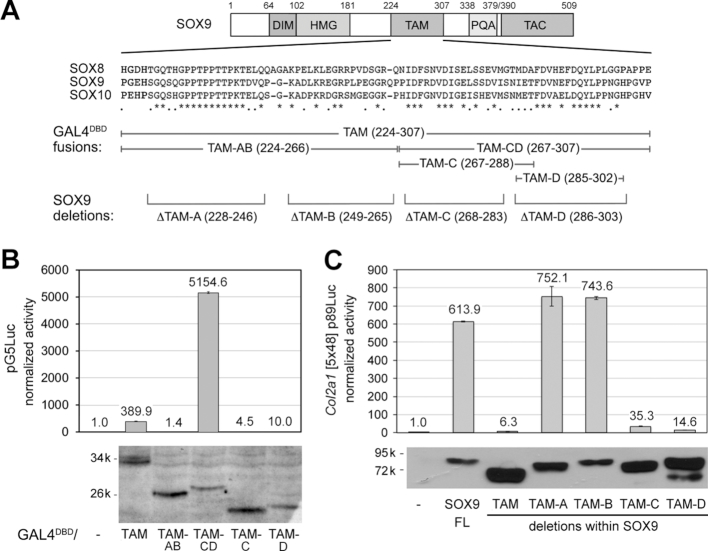
Figure 4.
Identification of subdomains of SOX9 TAM mediating transactivation. (A) From top to bottom, schematic of the SOX9 protein; alignment of the TAM sequences of the three human SOXE proteins; segments of TAM fused to GAL4DBD; and TAM-A to TAM-D sequences deleted in the SOX9 protein. (B) Reporter assay comparing the abilities of proteins made by fusing GAL4DBD with subdomains of SOX9 TAM to transactivate pG5Luc. Reporter activities are presented for one representative experiment as the mean ± standard deviation obtained for triplicate cultures per condition. Data were normalized for transfection efficiency and are reported as fold increase relative to the activity of the reporter in the presence of an empty expression plasmid. The western blot of cell lysates shows that all protein forms were efficiently made in the cells and thus that major differences in reporter activities among proteins genuinely reflect intrinsic differences in transactivation capabilities. These results were reproduced in multiple experiments. (C) Reporter assay comparing the abilities of wild-type SOX9 and SOX9 proteins lacking the whole TAM or TAM segments to transactivate the Col2a1 reporter. Reporter activities are presented as described in panel B. The western blot of cell lysates shows that all protein forms were efficiently made in the cells and thus that major differences in reporter activities among proteins genuinely reflect intrinsic differences in transactivation capabilities. These results were reproduced in multiple experiments.