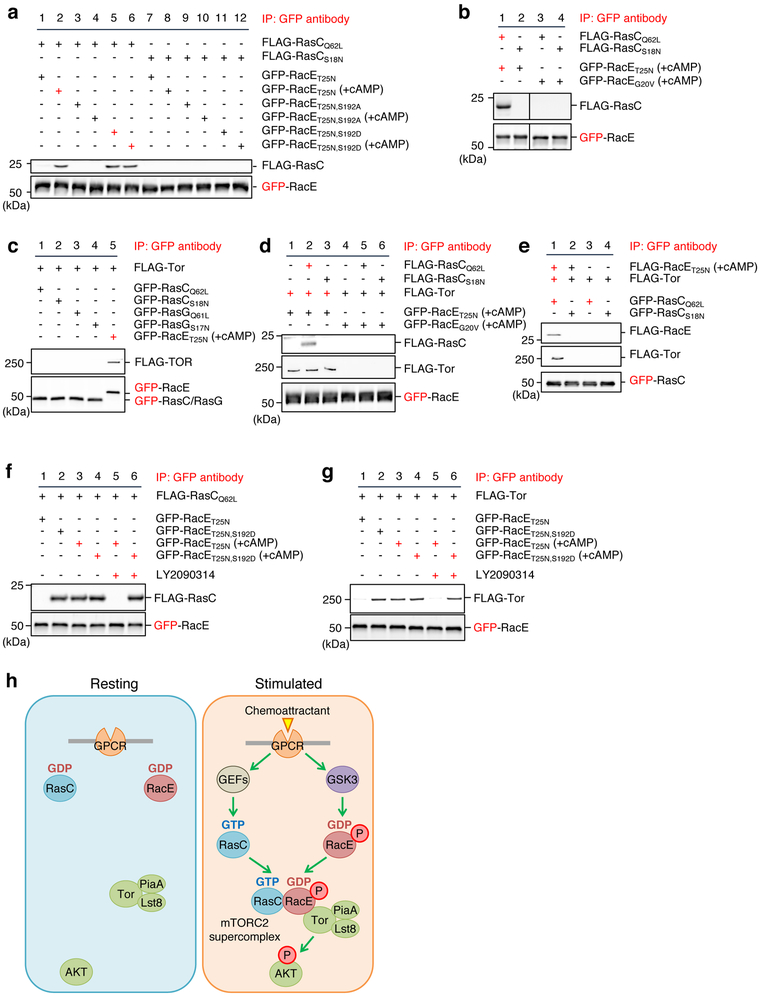
Figure 7. Ser192 phosphorylated RacE-GDP forms a supercomplex with Tor and Ras-GTP.
a and b, The indicated GFP-RacE proteins were purified from Dictyostelium cells with or without 1 μM cAMP stimulation for 30 s. FLAG-RasC proteins were purified without cAMP stimulation. GFP-RacE was incubated with FLAG-RasC and pulled down using GFP-Trap. The pellet fraction was analyzed by immunoblotting using antibodies to GFP and FLAG. c, GFP-RacE, GFP-RasC or GFP-RasG was incubated with FLAG-Tor that was purified in a high-salt condition and pulled down with GFP-Trap. The pellet fraction was analyzed by immunoblotting using antibodies to GFP and FLAG. d, GFP fused to GDP-bound RacET25N or GTP-bound RacEG20V were purified from Dictyostelium cells under a high salt condition after stimulation with the chemoattractant cAMP. These GFP fusion proteins were incubated with high-salt washed FLAG-Tor and/or FLAG-RasC proteins. GFP-RacE was pulled down with GFP-Trap, and the pellet fractions were analyzed by immunoblotting. e, RacE forms a complex with Tor and RasC. The indicated proteins were purified under high-salt conditions and mixed for 15 min at room temperature. GFP-RasC proteins were pulled down with GFP-Trap, and the pellet fraction was analyzed by immunoblotting. f and g, Different GFP-RacE proteins were purified from Dictyostelium cells with or without 1 μM cAMP stimulation for 30 s in the presence or absence of the GSK-3 inhibitor LY2090314 (250 nM). GFP-RacE was incubated with FLAG-RasC in f or FLAG-Tor in g and pulled down using GFP-Trap. The pellet fraction was analyzed by immunoblotting using antibodies to GFP and FLAG. h, Model for GPCR-mediated mTORC2-AKT signaling. In response to GPCR activation by chemoattractant, Rho-GDP becomes phosphorylated by GSK-3 and assembles the super signaling complex with Ras-GTP and mTORC2 to promote AKT phosphorylation. Experiments were repeated independently three times with similar results in a-g.