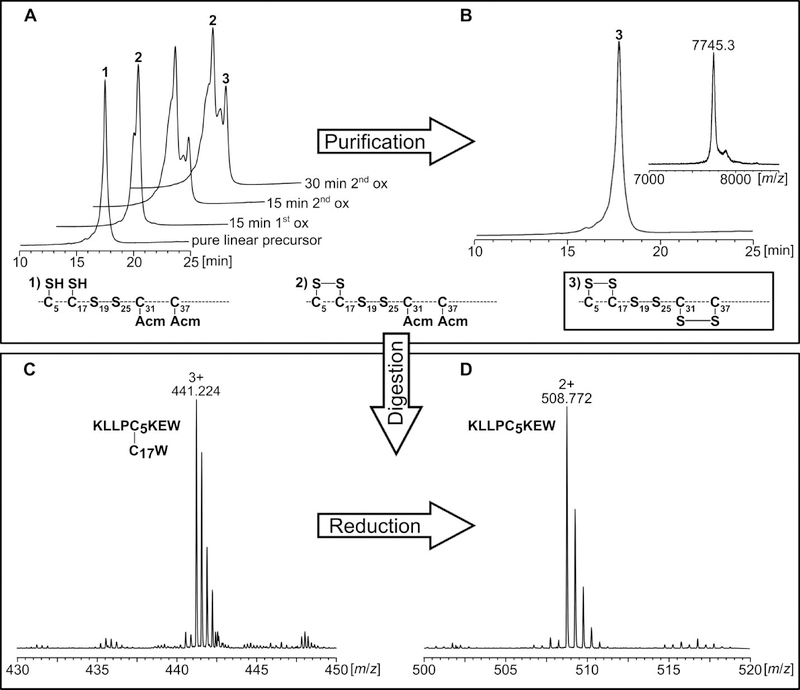
Figure 2.
Analysis of A[C19S, C25S] during synthesis (A, B) and chymotryptic digest for mass spectrometry analysis (C, D). A) Reaction control during first and second oxidation analyzed by HPLC. B) MS and HPLC analysis of purified A[C19S, C25S]. C) The chymotryptic digest of A[C19S, C25S] was analyzed by HPLC and the collected fractions were subjected to MS analysis. Peptide fragments containing disulfide bonds were identified as exemplified here for the C5-C17 bond (C, D). D) The fractions containing disulfide bonds were reduced with DTT, and the identity of the reduced fragments was confirmed by subsequent tandem mass spectrometry (example: reduced fragment containing C5).