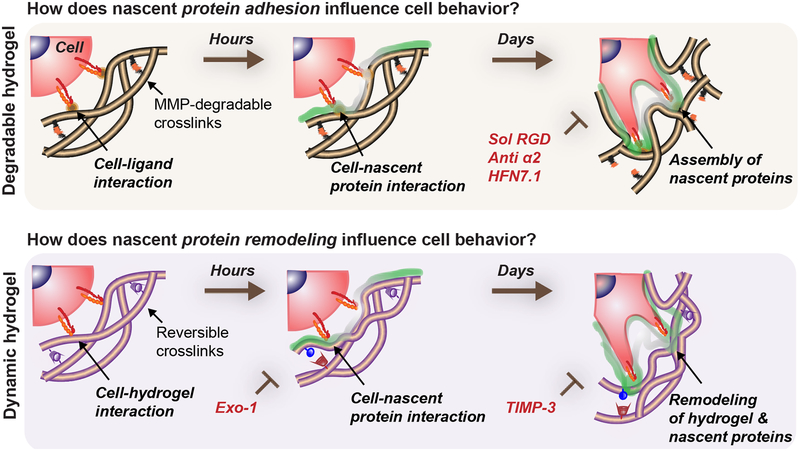
Figure 6. Nascent protein adhesion and remodeling enhance cell spreading in degradable/dynamic hydrogels.
Cells interact with a 3D hydrogel and presented ligands for a short time period before depositing nascent proteins to form a pericellular matrix. The hydrogel properties determine if encapsulated cells can spread, but adhesion and active remodeling of the nascent proteins are required for spreading. For example, in hydrogels that cells can locally degrade (e.g., protease-sensitive crosslinkers), nascent proteins guide cell behavior as an assembled interfacial layer that cells adhere to. Perturbation of cell-ECM adhesion through inhibiting specific cell-nascent protein interactions (e.g., sol RGD, anti α2, HFN7.1) inhibits spreading and decreases its downstream cellular outcomes (YAP/TAZ nuclear translocation, osteogenic differentiation). Similarly, in dynamic microenvironments (e.g., viscoelastic hydrogels) where spreading is protease-independent, nascent protein deposition and remodeling are needed for mechanosensing (YAP/TAZ nuclear translocation, osteogenic differentiation) and are blocked by inhibiting nascent protein secretion (Exo-1) and remodeling (TIMP-3).