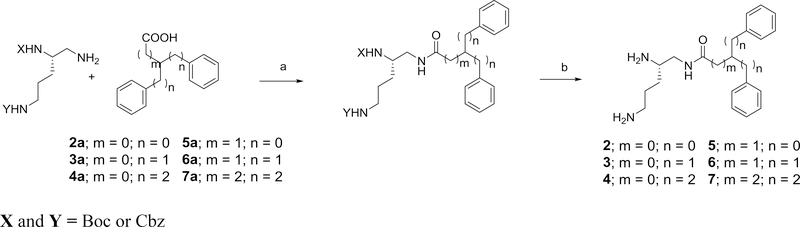
Scheme 2.
General method for forming the amide derivatives 2–7 using N’,N”-diprotected 2,5-diaminopentylamine and the appropriate acid. Reagents and conditions: a) EDC, HOBT, 2,6-lutidine; b) one step deprotection (20% Pd(OH)2/C, H2, ethanol) or a 2-step deprotection (i)TFA, DCM; ii) 20% Pd(OH)2/C, H2, ethanol).