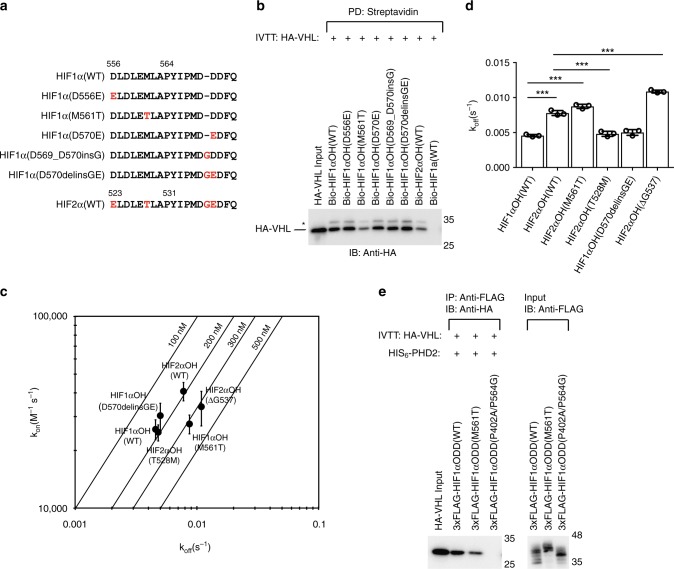
Fig. 2.
HIF1α Metn-3 stabilizes interaction with VHL. a Peptide sequences used in subsequent experiments are listed. Peptides are N-terminal biotinylated. Numbering is provided for the N-terminal amino acid residue and the proline hydroxylation site. b Biotinylated HIFαOH peptides were immobilized on streptavidin- agarose beads and incubated with in vitro transcribed and translated (IVTT) HA-VHL. Streptavidin beads were pulled down (PD) and levels of HA-VHL were visualized via immunoblotting (IB). c, d Biolayer interferometry kinetic analysis of VHL-elongin B-elongin C (VBC) complex binding to immobilized HIFα peptides. Biotinylated peptides were immobilized on streptavidin- coated biosensors and binding to VBC complex was monitored. The data was analyzed assuming a 1:1 binding model via the BLItz Pro software. c Rate plane with Isoaffinity Diagonals (RaPID) plot highlighting the kinetic parameters of VBC complex binding to HIFα peptides. Values represent mean of three experiments conducted with independently purified protein ± s.e.m. d The dissociation constants associated with VBC binding to HIFα peptides are shown on a linear scale. Statistical significance was assessed using a one-way ANOVA with Tukey’s post hoc test. Values represent mean of three experiments conducted with independently purified protein ± s.d. *** p < 0.005. e 3xFLAG-HIF1α oxygen-dependent degradation (ODD) domains were IVTT and incubated with purified HIS6-PHD2 (181–426). Following hydroxylation (one hour), 3xFLAG-HIF1α ODD domain was immobilized on protein A beads coated with anti-FLAG antibody and incubated with IVTT HA-VHL. 3xFLAG-HIF1α ODD domain was immunoprecipitated (IP) and levels of HA-tagged VHL were visualized via immunoblotting (IB). Molecular weight markers (kDa) are labeled for western blots