Fig. 5.
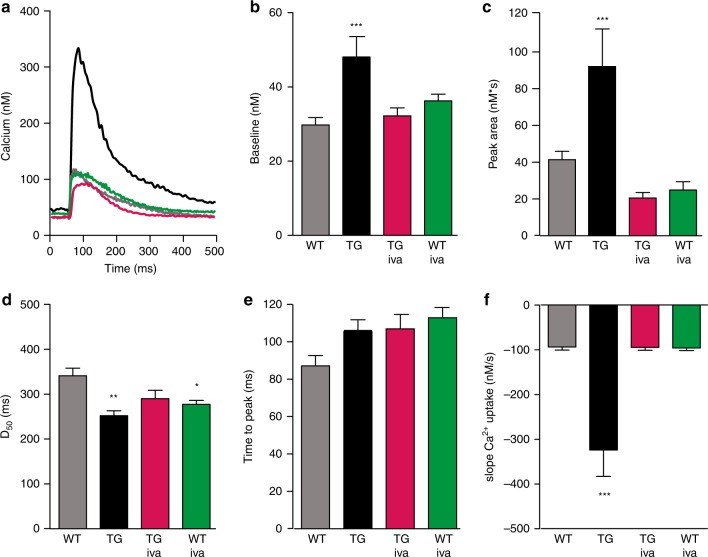
HCN4 overexpression affects intracellular Ca2+ homeostasis. a Representative [Ca2+]i transient traces measured as change in the Fluo-4 fluorescence in electrically driven cardiomyocytes from wild type [baseline (gray) and ivabradine treated (green)] and HCN4tg/wt [baseline (black) and ivabradine treated (magenta)] mice (3 months of age). b–f Quantitative analysis reveals a significant change of [Ca2+]i to higher diastolic baseline levels (b), and larger amounts of total cellular Ca2+ movement (reflected by the area under the curve) during the systole in HCN4tg/wt cardiomyocytes (c). Similar time to peak among groups indicates unaffected Ca2+ release from sarcoplasmic reticulum (SR) by ryanodine receptor (RyR) activity (d). Quantification of time to half decay of Ca2+ transients (D50) (e), and slope Ca2+ uptake (f), show that removal of increased baseline [Ca2+]i to the SR, driven by SERCA, is augmented in HCN4tg/wt cardiomyocytes. Changes of HCN4tg/wt cardiomyocytes in Ca2+ homeostasis were mostly reversed by treatment with the If blocker ivabradine (3 µM) underlining that the overexpressed HCN4 current is the pivotal cause for the observed Ca2+ imbalance. Data are expressed as mean ± s.e.m. (wild type: n = 90 cells, HCN4tg/wt: n = 42 cells, iva-treated wild type: n = 48 cells, iva-treated HCN4tg/wt: n = 75 cells derived from six animals/group; *P < 0.05, **P < 0.01, ***P < 0.001 compared to wild type; ANOVA followed by Tukey test). Source data are provided as a Source data file