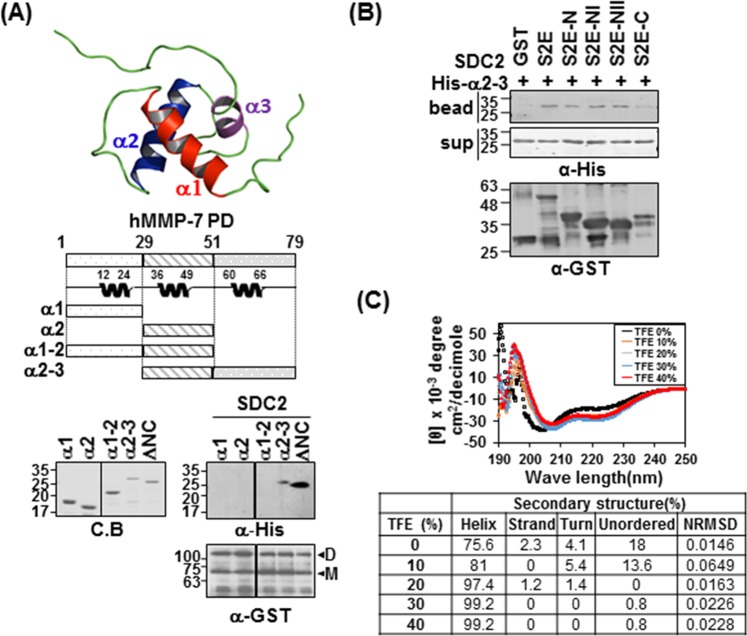
Figure 4.
The α2–3 helix of the MMP-7 pro-domain is involved in its interaction with the syndecan-2 extracellular domain. (A) Structure of the MMP-7 pro-domain (PDB; 2MZE) and schematic diagram of the MMP-7 pro-domain (PD) and its deletion mutants. The indicated MMP-7 pro-domain mutants were purified with Ni-NTA agarose beads, separated by SDS-PAGE. Purified MMP-7 pro-domain mutants were incubated with GST-SDC-2. Materials bound to glutathione-agarose beads were immunoblotted with an anti-His tag antibody and the membranes were stripped and re-probed with an anti-GST antibody. Image is of a single membrane cropped to remove intervening lanes. (B) Purified GST-SDC2 mutants were incubated with His-tagged α2–3 helix of the MMP-7 pro-domain. Materials bound to glutathione-agarose beads were immunoblotted with an anti-His tag antibody (top) and the membranes were then stripped and re-probed with an anti-GST antibody (bottom). (C) The secondary structure of the α2–3 helix of the MMP-7 pro-domain was analyzed using CD in the presence of different concentrations of TFE (top). Quantitative estimations of the secondary-structure content were made with the CDPro software package, which includes the programs CDSSTR, CONTIN, and SELCON3. The α-helical fractions were extracted from the CDPro calculations based on empirical methods with ellipticities set at 208 or 222 nm (bottom). Smaller values of NRMSD indicate closer correspondence between calculated structures and the experimental data.