FIGURE 7.
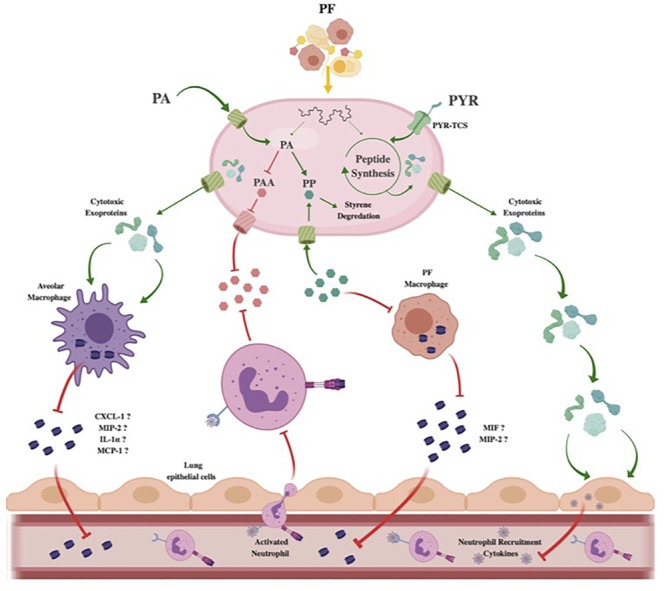
Theoretical model of Acinetobacter baumannii neutrophil evasion strategy elicited by PF. In the host respiratory space, A. baumannii encounters PF, inducing a survival-based transcriptional response. PA uptake and alternative catabolism toward styrene degradation are amplified to prevent PP accumulation. The halting of PP release inhibits PF-resident macrophages from utilizing the phenylpyruvate tautomerase cytokine macrophage inhibitory factor (MIF). Lack of active MIF reduces mobilization of MIP-2, a known neutrophil recruiting cytokine. Concurrently, the PAA catabolic route for PA degradation is downregulated to prevent bacterial metabolite-driven neutrophil chemoattraction. Resident respiratory space macrophages (pictured as alveolar macrophages) are then targeted by secreted cytotoxic exoproteins induced by PF, preventing pathogen recognition and subsequent secretion of proinflammatory chemokines such as CXCL-1, MIP-2, IL-1α, and MCP-1. Simultaneously, host-derived PYR is sensed by a membrane two-component system transport protein (PYR-TCS) to be used in conjunction with the effects of PF to produce epithelial cell-specific cytotoxic proteins. Epithelial cell (pictured as lung epithelial cells) inflammatory response is then reduced, inhibiting second-wave neutrophil recruitment. Red inhibitory lines represent downregulated/inhibited processes. Green arrows and proteins represent upregulated processes.
