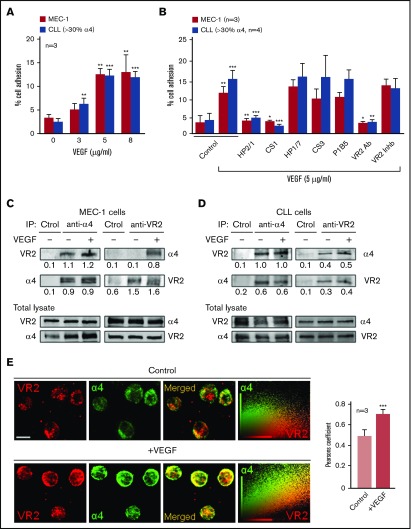
Figure 1.
Functional association between α4β1 integrin and VEGFR2 in CLL cells. (A) 2′,7′-Bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester (BCECF-AM)–labeled MEC-1 cells (3 independent experiments) or primary CLL cells from 3 patients (P4, P9, P10) were added to wells coated with the indicated concentrations of VEGF. After 60 minutes at 37°C, attached cells were quantified using a fluorescence analyzer. (B) MEC-1 cells (3 independent experiments) or primary CLL cells (P1, P4, P5, P9), with or without previous incubation with the indicated inhibitors, were added to wells coated with 5 μg/mL VEGF and adhesion was quantified as explained. Values represent the percentage of the total number of cells added. (C-D) A total of 15 × 106 MEC-1 cells (C) or primary CLL cells (D; P4) were serum-starved for 2 hours and treated or not with 50 ng/mL soluble VEGF for 15 minutes. Cells were lysed, immunoprecipitated (IP) with anti-α4, anti-VEGFR2 (VR2), or control (Ctrol) Abs and analyzed by western blotting. The total lysate for each condition was also analyzed by western blotting. Numbers indicate the ratio of immunoprecipitated protein with respect to the amount of that protein in the total lysate. (E) Primary CLL cells (P2, P5, P11) were cultured (2 hours, 37°C) on glass coverslips coated with 10 μg/mL poly-l-lysine (control, top panels) or 5 μg/mL VEGF (bottom panels). Cells were fixed and analyzed by confocal microscopy using the indicated primary Abs and Alexa 568– or Alexa 488–labeled secondary Abs. Colocalization of α4 integrin (green) and VEGFR2 (red) was further demonstrated by dot-plot analyses and quantified by the Pearson correlation coefficient. Scale bar, 4 µm. Confocal images for P11 and average values for the 3 patients analyzed ± standard error of the mean (SEM) are shown. *P < .05; **P ≤ .01; ***P ≤ .001.