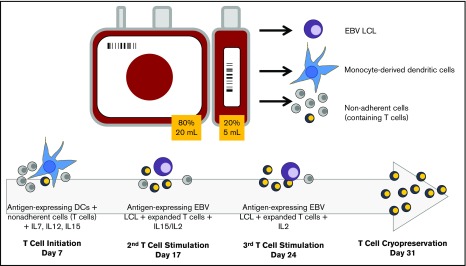
Figure 1.
Schematic of CB-VST manufacture in ACT-CAT and ACTCAT2 clinical trials. Eighty percent of the CB unit was used as the CB transplant graft, and 20% was used to expand the VST. From the 20% fraction, 5 to 10 million peripheral blood mononuclear cells (PBMCs) were used for EBV lymphoblastoid cell line (LCL) generation; the remaining cells were used to generate dendritic cells (DCs) or used as the source of T cells. DCs were matured and transduced with the Ad5f35-pp65 vector (ACT-CAT) on day 5 and/or cocultured with overlapping viral peptides (PepMix) (ACT-CAT2), then cocultured with the nonadherent fraction (containing T cells) on day 7 in the presence of IL-7, IL-12, and IL-15. At the second and third stimulation, EBV-transformed B cells were transduced with the same Ad5f35-pp65 vector 2 days before the T-cell stimulation or pulsed with PepMix the day of stimulation, which was performed in the presence of IL-15 (second stimulation) and IL-2 (third stimulation).