Figure 28.
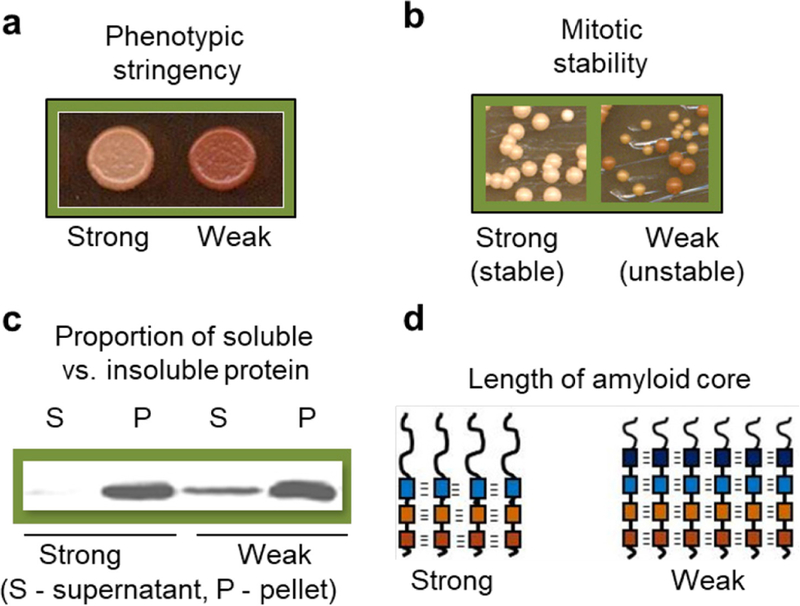
Molecular basis of prion/amyloid strains. (a) Phenotypic stringency of the strong and weak strains of yeast prion protein Sup35, as indicated by color on complete medium (stronger prion phenotype is associated with less accumulation of a red pigment, leading to a lighter color). (b) Differences in mitotic stability between strong and weak prion strains of Sup35 protein (mitotic loss of a prion leads to generation of red colonies). (c) Differences in proportion of aggregated (P, pellet) and non-aggregated (S, supernatant) protein between extracts of yeast cells bearing the strong and weak strains of the Sup35 prion, as demonstrated by differential centrifugation, followed by SDS-PAGE and reaction to Sup35 antibodies. (d) Differences in the length of amyloid core between the strong and weak strains of the Sup35 prion. Presumable β-strands are schematically indicated by boxes, and hydrogen bonds by dashes.
