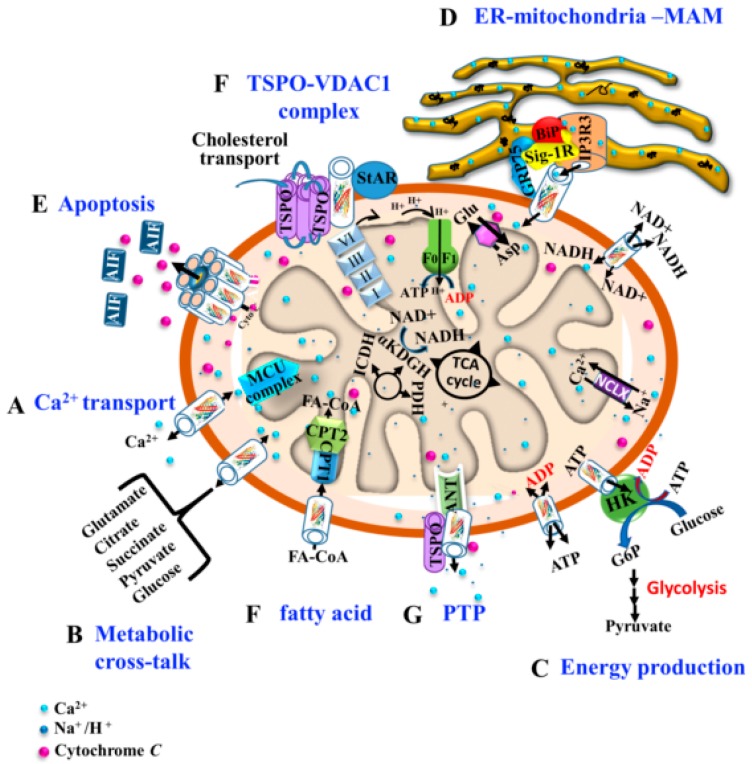
Figure 1.
Schematic representation of VDAC1 as a multi-functional protein involved in Ca2+ and metabolite transport, energy production and the structural and functional association of mitochondria with the ER. The various functions of VDAC1 in cell and mitochondria functions are presented. These include: (A) Transporting Ca2+ across the OMM, thereby modulating Ca2+ signaling. In the IMM, Ca2+ uptake into the matrix is mediated by a Ca2+-selective transporter, the mitochondrial Ca2+ uniporter (MCU), regulated by a calcium-sensing accessory subunit (MCU1). Ca2+ efflux is mediated by NCLX, a Na+/Ca2+ exchanger. Ca2+ controls energy production via activation of PDH, ICDH, and α KGDH by intra-mitochondrial Ca2+, leading to enhanced activity of the citric acid cycle; (B) Control of metabolic cross-talk between the mitochondria and the rest of the cell, by transporting metabolites; (C) Mediating cellular energy production by transporting ATP/ADP and NADH and acyl-CoA from the cytosol to the IMS, and regulating glycolysis via the association with HK; (D) Involvement in structural and functional association with the ER, mediating Ca2+ transport from the ER to mitochondria. Key proteins, such as the inositol 3 phosphate receptor type 3 (IP3R3), the sigma1 receptor (Sig1R), the chaperone HSP70, and glucose-regulated protein 75 (GRP75) are presented; (E) Participation in apoptosis via its oligomerization to form a protein-conducting channel, allowing Cyto c release and cell death; and (F) Mediation of the transfer of fatty acid acyl-CoAs across the OMM to the IMS, where they are converted into acylcarnitine by CPT1a for further processing by β-oxidation. VDAC1 is involved in cholesterol transport as a constituent of a multi-protein complex, the transduceosome, containing Star, TSPO and VDAC1. (G) The permeability transition pore (PTP), composed of VDAC at the OMM, ANT at the IMM and Cyp D in the matrix, allows release of apoptogenic proteins.