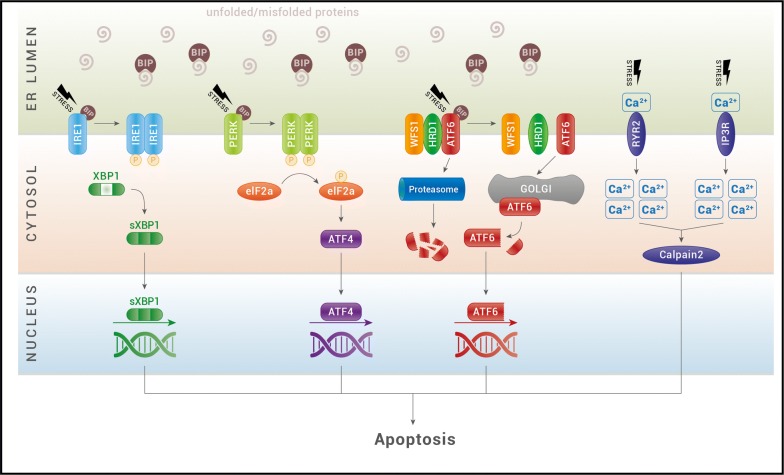
Fig. 1.
The ER stress pathway. Under situations of stress, unfolded and misfolded proteins accumulate and recruits BIP to the ER lumen. BIP dissociates from the ER stress sensors IRE1α (inositol-requiring protein 1), ATF6 (activating transcription factor 6) and PERK [protein kinase RNA (PKR)-like ER kinase] and leads to their activation. Upon dimerization and autophosphorylation, IRE1 induces the splicing of XBP1 mRNA for translation of the transcription factor spliced XBP1 protein (sXBP1). XBP1s translocates to the nucleus and controls the transcription of ER-resident chaperones, components of the ERAD machinery and genes involved in lipogenesis. Activated PERK causes the phosphorylation of eukaryotic initiation translation factor 2α (eIF2α), which increases production of activating transcription factor 4 (ATF4). ATF4 then translocates to the nucleus and induces the transcription of many genes required for ER quality control. Activated ATF6 translocates to the Golgi, where it is processed by S1P and S2P proteases. The cleaved-off cytoplasmic domain functions as a transcription factor and induces the expression of ER chaperones and XBP1. ATF6 activity is inhibited by the WFS1 protein, that through the E3 ubiquitin ligase HRD1, is responsible of ATF6 ubiquitin-mediated proteasomal degradation. ER calcium channels, ryanodine receptor (RyR) and inositol triphosphate receptor (IP3R), control efflux of calcium (Ca2+) from the ER to the cytosol. Under ER stress activation, these receptors increase the levels of cytosolic calcium and activate the calcium-dependent protease, calpain-2, which promotes cellular apoptosis