Abstract
Background:
Studies have suggested that micronutrient deficiency has some role in the progression of chronic heart failure (CHF).
Hypothesis:
Oral supplementation with coenzyme Q10 (CoQ10) and creatine may reduce mitochondrial dysfunction that contributes to impaired physical performance in CHF.
Methods:
We conducted a randomized, double‐blind, placebo‐controlled trial to determine the effect of a mixture of water‐soluble CoQ10 (CoQ10 terclatrate; Q‐ter) and creatine on exercise tolerance and health‐related quality of life. Exercise tolerance was measured as total work capacity (kg·m) and peak oxygen consumption (VO2, mL/min/kg), both from a cardiopulmonary exercise test. Health‐related quality of life was measured by the Sickness Impact Profile (SIP) in CHF secondary to left ventricular systolic dysfunction (left ventricular ejection fraction ≤ 35%). After baseline assessment, 67 patients with stable CHF were randomized to receive Q‐ter 320 mg + creatine 340 mg (n = 35) or placebo (n = 32) once daily for 8 weeks.
Results:
At multivariate analysis, 8‐week peak VO2 was significantly higher in the active treatment group than in the placebo group (+1.8 ± 0.9 mL/min/kg, 95% CI: 0.1–3.6, P < 0.05). No untoward effects occurred in either group.
Conclusions:
This study suggests that oral Q‐ter and creatine, added to conventional drug therapy, exert some beneficial effect on physical performance in stable systolic CHF. Results may support the design of larger studies aimed at assessing the long‐term effects of this treatment on functional status and harder outcomes. © 2011 Wiley Periodicals, Inc.
The study was supported by an unconditional grant from Scharper Therapeutics, Milan, Italy, which had the responsibility for manufacturing the active treatment and the placebo. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
See Editorial on Page 196
Introduction
Chronic heart failure (CHF) is a major health problem whose incidence increases exponentially with age.1 As a result of the aging population and of improved treatment of acute coronary syndromes, the prevalence of CHF has also dramatically increased in recent years.1, 2, 3 Beyond being a cause of increased mortality, hospital readmissions, and medical visits, CHF is associated with disability,4 reduced exercise tolerance,5 and impaired health‐related quality of life (HRQL).6, 7
A deficiency of micronutrients and vitamins may contribute to the progression of CHF; and, reciprocally, CHF patients may become deficient in micronutrients due to reduced intake and increased wasting secondary to both cachexia and diuretic therapy.8 Supplementation of micronutrients has been proven of some utility in the prevention of skeletal muscle myopathy, which is an important contributor to impaired physical performance associated with CHF.9
It has been suggested that oral supplementation of coenzyme Q10 (CoQ10), a key antioxidant synthesized by human cells,10 may have beneficial effects in CHF through its capacity to reduce oxidative stress and improve energy production in the mitochondria.11 However, because of limited solubility in water, CoQ10 is characterized by poor bioavailability and chemical instability.12
Creatine is a micronutrient whose dietary supplementation has been shown to increase exercise performance and fat‐free mass in normal individuals.13 Creatine and its phosphorylated form, phosphocreatine,13 have been proven to protect against adenosine triphosphate (ATP) depletion, stimulate protein synthesis, reduce protein degradation, and stabilize biological membranes.13
The present randomized, double‐blind, placebo‐controlled trial was aimed at evaluating the effects of oral supplementation of a commercially available combination of water‐soluble CoQ10 (CoQ10 terclatrate; Q‐ter)14 and creatine (Eufortyn; Scharper Therapeutics, Milan, Italy) on exercise tolerance and HRQL in patients with stable CHF secondary to left ventricular (LV) systolic dysfunction.
Because of multiple concurrent metabolic alterations in cardiac and skeletal muscular cells in CHF,15 supplementation of a single factor might be insufficient to restore energy‐production pathways.15 The Q‐ter supplement is a multicomposite consisting of a mixture of copovidone (acting as a carrier), CoQ10, and glycine (works as a catalyst). Compared with native CoQ10, Q‐ter is about 200× more soluble in water, while retaining its antioxidant capacity.16, 17 This results in greater bioavailability and chemical stability compared with the native form (G. Manini, Scharper Therapeutics; personal communication).
Q‐ter is manufactured using an industrially available native CoQ10 (Kaneka Pharma Europe, Brussels, Belgium).
Methods
Patient Selection
All patients with CHF due to LV systolic dysfunction and LV ejection fraction (LVEF) ≤35% consecutively evaluated at our CHF outpatient clinic between January 2007 and December 2008 were considered eligible for the study, provided they had been clinically stable during the last 6 months while on standardized, guideline‐based medical therapy.1 Exclusion criteria were: (1) idiopathic hypertrophic, alcoholic, or toxic cardiomyopathy; (2) recent (<3 months) acute coronary syndromes, cardiac surgery, or percutaneous coronary intervention; (3) ventricular arrhythmias requiring ICD implantation; (4) atrial fibrillation with uncontrolled heart rate; (5) contraindications to physical exercise testing (chronic obstructive pulmonary disease requiring oxygen therapy; chronic renal failure with creatinine >2.5 mg/dL; anemia with hemoglobin <10 g/dL)18; (6) indication for heart transplantation; (7) active cancer; (8) participation in rehabilitation programs during the previous 6 months; and (9) unwillingness/inability to sign a written, informed consent.
Study Design
A preliminary, open‐label, placebo‐controlled study was performed in 4 patients with stable CHF. Of these, 2 patients were given 2 oral doses of Eufortyn powder once a day for 1 month, each dose containing 160 mg of Q‐ter (equivalent to 16 mg of native CoQ10)14 and 170 mg of creatine. The other 2 patients were given a placebo. Active treatment was associated with a 9 ± 2% increase in total work capacity (TWC; kg·m), assessed from symptom‐limited cardiopulmonary bicycle exercise testing, compared with no change with placebo. Using these data, we calculated that 27 patients for each group would be necessary to detect a similar increase in TWC associated with active treatment (α = 0.05 and power = 0.80). Anticipating a dropout rate of 10% and a possible slight improvement due to adaptation to repeated testing also in the placebo group, the sample size was conservatively increased to 35 patients in each group.
At baseline, patients underwent a symptom‐limited cardiopulmonary bicycle exercise test, with load increases of 60 kg·m (ie, 10 watts) every minute. Analysis of expired gas was performed with a metabolic cart equipment (Ultima CPX; Medical Graphics Corp., St. Paul, MN) calibrated before each test. Minute ventilation, oxygen uptake indexed by body weight (mL/min/kg), and carbon dioxide output were acquired on a breath‐by‐breath basis. Peak oxygen consumption (VO2) was taken as the average of the measures obtained over the last 30 seconds of the maximal workload attained. Standard 12‐lead electrocardiography and blood pressure readings were recorded at rest and then every minute during exercise and recovery phases. Health‐related quality of life was measured with a validated Italian version of the Sickness Impact Profile (SIP),19 a generic, self‐reported instrument consisting of 136 items, whose total score and physical and psychosocial subscores increase with worsening HRQL.
After baseline assessment, patients were randomized to receive either active treatment or placebo. The randomization list remained concealed to the investigators; both patients and physicians, who were in charge of the enrollment and assessment, were blinded toward assignment. Active treatment consisted of an oral dose of the Eufortyn Q‐ter and creatine combination (320 mg and 340 mg, respectively) taken once a day for 2 months. Control patients were given oral placebo once a day for the same period of time.
Exercise tolerance and HRQL were then reassessed the day after completion of the treatment period. To check adherence to treatment, on that occasion patients were invited to bring their medication package, and residual doses were counted.
The study protocol was approved by the local ethics committee, and each patient gave written informed consent.
Study Endpoints
Improvement in physical performance as determined by changes in TWC and peak VO2 was the primary endpoint of the study; treatment effects on HRQL represented the secondary endpoint.
Statistical Analysis
Statistical analysis was performed with SPSS for Windows, version 17.0 (SPSS Inc., Chicago, IL). Continuous and categorical variables were expressed as mean ± SD and percentages, respectively. At baseline, continuous variables were compared between treatment groups with the Student t test or, in case of non‐normal distribution, with the Mann‐Whitney test. The χ 2 test was used to analyze differences in categorical variables. The effects of active treatment and placebo over time were first assessed in univariate analysis with paired t test. Then, general linear regression models were built to analyze differences in TWC and VO2 between treatment groups at 8 weeks, while adjusting for potential confounders. In this analysis, the mean (±SE) difference between active treatment and placebo, and 95% confidence intervals, were calculated. A 2‐tailed P value <0.05 was considered statistically significant in all analyses.
Results
Patient Characteristics, Treatment Adherence, and Adverse Drug Reactions
Over the study period, 83 patients were screened for eligibility. However, before entering the randomization process, 16 (19%) potential candidates were excluded for medical reasons (worsening acute heart failure, n = 8; cardiogenic shock with indication for heart transplantation, n = 1; gastric ulcer, n = 1; femur fracture, n = 1; and lumbar vertebral body fracture, n = 1) or unwillingness to consent (n = 4). Finally, 67 patients were enrolled and randomly assigned to receive Q‐ter and creatine (n = 35) or placebo (n = 32).
As shown in Table 1, baseline clinical characteristics, including demographics, cardiovascular risk factors, and comorbidities, were similar in the 2 groups. Both groups included mostly men with ischemic heart disease as CHF etiology; the proportion of those with previous myocardial revascularization was also similar. Left ventricular ejection fraction was moderately to severely impaired in both groups; however, as shown by the large prevalence of patients in New York Heart Association (NYHA) class II, functional status was fairly preserved. Baseline medical therapy was similar in the 2 groups, with the exception of antialdosterone agents, which were slightly more frequently used by those randomized to placebo (P = 0.05) (Table 1). On average, drug treatment was more adherent to guidelines than usually seen in a real‐life older CHF population in registry studies.20 Baseline exercise tolerance and HRQL were also similar in the 2 groups (Table 1). Adherence to trial treatment, as evaluated by remaining dose count, was 100% in both groups. No adverse drug reactions were reported. All enrolled patients completed the study protocol, and baseline pharmacological therapy remained unchanged in all patients during the study period.
Table 1.
Demographic and Clinical Characteristics by Treatment Group
| Q‐ter + creatine (n = 35) | Placebo (n = 32) | P Value | |
|---|---|---|---|
| Age (y) | 72 ± 6 | 71 ± 6 | 0.38 |
| Male gender (n, %) | 29 (82.9) | 28 (87.5) | 0.75 |
| Weight (kg) | 73 ± 13 | 76 ± 12 | 0.34 |
| Height (m) | 1.69 ± 0.06 | 1.79 ± 0.07 | 0.65 |
| Current smokers (n, %) | 5 (14.3) | 4 (12.5) | 0.32 |
| COPD (n, %) | 3 (8.6) | 4 (12.5) | 0.70 |
| DM (n, %) | 13 (37.1) | 12 (37.5) | 1.00 |
| Dyslipidemia (n, %) | 19 (54.3) | 14 (53.7) | 0.20 |
| HT (n, %) | 19 (54.3) | 20 (62.5) | 0.62 |
| MI (n, %) | 24 (68.6) | 21 (65.6) | 0.50 |
| Myocardial revascularization (n, %) | |||
| PCI | 14 (40.0) | 9 (28.1) | 0.49 |
| CABG | 12 (34.3) | 11 (34.4) | |
| PAD (n, %) | 5 (14.3) | 7 (21.9) | 0.53 |
| Stroke (n, %) | 1 (2.9) | 2 (6.3) | 0.60 |
| AF (n, %) | 5 (14.3) | 8 (25.0) | 0.36 |
| CHF etiology (n, %) | |||
| ischemic | 29 (82.9) | 24 (75.0) | 0.55 |
| nonischemic | 6 (17.1) | 8 (25.0) | |
| LVEF (%) | 30 ± 4 | 31 ± 4 | 0.37 |
| NYHA class (n, %) | |||
| II | 31 (88.6) | 30 (93.8) | 0.68 |
| III | 4 (11.4) | 2 (6.3) | |
| Medical therapy (n, %) | |||
| ACEI/anti‐ATII | 32 (91.4) | 31 (96.9) | 0.62 |
| Antialdosteronic agents | 11 (31.4) | 18 (56.3) | 0.05 |
| β‐Blocking agents | 23 (65.7) | 24 (75.0) | 0.44 |
| Digoxin | 16 (45.7) | 12 (37.5) | 0.62 |
| Furosemide | 25 (71.4) | 29 (90.6) | 0.07 |
| Furosemide dose (mg/d)a | 25.0 (10.7–25.0) | 37.5 (25.0–75.0) | 0.07 |
| Total work capacity (kg·m) | 2118 ± 1118 | 2013 ± 936 | 0.68 |
| Peak VO2 (mL/kg/min) | 13.4 ± 2.3 | 12.8 ± 2.6 | 0.29 |
| SIP total score | 8.3 ± 7.4 | 9.0 ± 5.6 | 0.67 |
| SIP physical score | 6.5 ± 5.1 | 6.7 ± 6.3 | 0.86 |
| SIP psychosocial score | 7.8 ± 7.0 | 10.0 ± 8.6 | 0.25 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; anti‐ATII, angiotensin II type 1 receptor antagonist; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HT, hypertension; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; PAD, peripheral artery disease; PCI, percutaneous coronary intervention (primary or elective angioplasty); Q‐ter, coenzyme Q10 terclatrate; SIP, Sickness Impact Profile; VO2, oxygen consumption.
Furosemide dose represents median daily dose of furosemide (33rd–66th percentile).
Treatment Effects
After 8 weeks, TWC significantly improved in patients receiving Q‐ter and creatine (2118 ± 1118 kg·m at baseline vs 2322 ± 1272 kg·m at 8 weeks, +10.4%, P = 0.04), whereas it was not significantly changed in those receiving placebo (2013 ± 936 kg·m at baseline vs 2125 ± 1022 kg·m at 8 weeks, P = 0.06) (Figure 1A). Similarly, peak VO2 increased in the active treatment group (13.4 ± 2.3 mL/min/kg at baseline vs 14.4 ± 2.9 mL/min/kg at 8 weeks, +7.5%, P = 0.003) and was unchanged in the placebo group (12.8 ± 2.6 mL/min/kg at baseline vs 13.2 ± 2.5 mL/min/kg at 8 weeks, P = 0.108) (Figure 2A).
Figure 1.
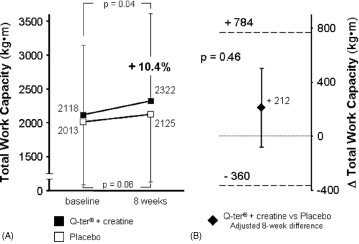
Univariate analysis of changes in TWC (A) after 8 weeks of oral supplementation of Q‐ter + creatine (black boxes) or placebo (open boxes). Change from baseline was significant only with active treatment. (B) Multivariate analysis of mean difference (±SE) in TWC at 8 weeks between treatment groups (Q‐ter + creatine vs placebo), adjusted for diabetes. Dashed lines represent the 95% confidence intervals of the estimate. Lower standard error bar across the 0 line indicates no difference in TWC between the 2 groups. Abbreviations: Q‐ter, coenzyme Q10 terclatrate; TWC, total work capacity.
Figure 2.
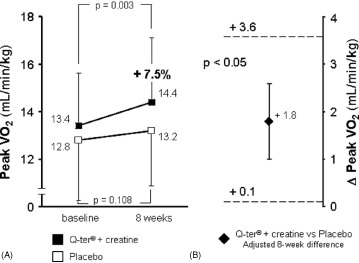
Univariate analysis of changes in peak VO2 (A) after 8 weeks of oral supplementation of Q‐ter + creatine (black boxes) or placebo (open boxes). Change from baseline was significant only with active treatment. (B) Multivariate analysis of mean difference (±SE) in peak VO2 at 8 weeks between treatment groups (Q‐ter + creatine vs placebo), adjusted for peripheral artery disease (see text). Dashed lines represent the 95% confidence intervals of the estimate. Lower standard error bar above 0 indicates that active treatment produced a greater peak VO2 compared with placebo. Abbreviations: Q‐ter, coenzyme Q10 terclatrate; VO2, oxygen consumption.
Among clinical variables, only diabetes mellitus (P = 0.032) and peripheral artery disease (P = 0.004) were univariate predictors of reduced 8‐week improvement in TWC and peak VO2, respectively. In multivariate analysis, after adjustment for the presence of peripheral artery disease, 8‐week peak VO2 was significantly greater in patients treated with Q‐ter and creatine than in controls (Figure 2B); on the contrary, after adjusting for the presence of diabetes, the difference in TWC between groups was not significant (Figure 1B). The slightly different use of antialdosterone agents between the 2 groups did not affect either of the 2 models.
Univariate analysis showed that SIP total score and psychosocial subscore were unchanged in both study arms, whereas SIP physical subscore significantly improved only in patients receiving the active treatment (Table 2). The 8‐week SIP physical subscore, however, was not significantly different between the 2 groups. No other clinical variables exerted any influence on SIP score changes.
Table 2.
Changes in Health‐Related Quality of Life by Treatment Group
| Baseline | 8 Weeks | P Value | |
|---|---|---|---|
| SIP total score | |||
| Q‐ter + creatine | 8.4 ± 7.4 | 7.7 ± 8.1 | 0.44 |
| Placebo | 9.1 ± 5.6 | 8.2 ± 6.3 | 0.09 |
| SIP physical score | |||
| Q‐ter + creatine | 6.5 ± 5.1 | 5.1 ± 6.5 | <0.01 |
| Placebo | 6.8 ± 6.4 | 5.9 ± 5.6 | 0.21 |
| SIP psychosocial score | |||
| Q‐ter + creatine | 7.8 ± 7.0 | 7.5 ± 7.3 | 0.78 |
| Placebo | 10.0 ± 8.6 | 9.0 ± 9.9 | 0.18 |
Abbreviations: Q‐ter: coenzyme Q10 terclatrate; SIP, Sickness Impact Profile.
Discussion
This randomized, double‐blind, placebo‐controlled trial showed that, in patients with stable CHF, a 2‐month oral supplementation of a commercially available combination of Q‐ter and creatine produced a significant improvement in exercise tolerance and in HRQL, as estimated by the SIP physical component.
Previous reports indicated a clinically protective effect of nutraceuticals (ie, normal components of foods, delivered for therapeutic purposes in concentrations higher than what is available in a normal diet) in CHF patients, attributed to their ability to modulate several metabolic pathways that are altered in heart failure,21 such as mitochondrial energy depletion, oxidative stress, and calcium overload. These metabolic derangements result in alterations of the cytoskeleton, development of excitotoxicity (ie, acute cell injury after intense receptor activation), cellular dysfunction, apoptosis, and necrosis. Mitochondrial dysfunction is the key element of these processes, with defective energy production and increased oxidative stress that contribute to alter the structure of DNA, proteins, and lipids.21 Both CoQ10 and creatine can positively interfere with these mechanisms.
CoQ10 Effects
CoQ10 is an endogenous, vitamin‐like, fat‐soluble quinine, highly concentrated in myocardium, liver, and kidney mitochondria.22 CoQ10 is an electron carrier involved in the synthesis of ATP; it has membrane‐stabilizing properties and is a powerful antioxidant.11, 22 Mitochondria from patients with CHF are depleted of CoQ10,22 and it has been recently demonstrated that a reduced plasma concentration of the molecule is an independent predictor of increased mortality in CHF patients.23 However, individual randomized controlled trials aimed at evaluating the effects of CoQ10 in patients with CHF have obtained variable, and sometimes conflicting, results.12 In a randomized, double‐blind, controlled trial, the daily administration of 200 mg of CoQ10 for 6 months to patients in NYHA class III‐IV failed to improve LVEF, peak VO2, and exercise tolerance.24 Conversely, a single‐center, randomized, placebo‐controlled, crossover trial demonstrated that a daily dose of 300 mg of CoQ10 for 4 weeks, in patients with less‐severe CHF (NYHA class II‐III), produced a remarkable improvement in both LV contractility and peak VO2.25, 26 In addition, 2 meta‐analyses of 827 and 1128 randomized trials, respectively, concluded that oral supplementation of CoQ10 for a period of 1–6 months was associated with significant improvements in LVEF and cardiac output. Based on these results, the still‐in‐progress Q‐SYMBIO international, multicenter, double‐blind trial has been specifically designed to test the effects of CoQ10 on cardiovascular morbidity and mortality in CHF patients.29
Creatine Effects
Creatine is a phosphate donor that is essential to maintain high levels of intracellular ATP.22 In the mitochondrion, creatine kinase catalyzes the transfer of high‐energy phosphates from creatine phosphate to adenosine diphosphate, thereby maintaining high ATP concentrations.13, 30 Studies suggest that creatine phosphate concentration is reduced in the failing myocardium and that the creatine phosphate/ATP ratio is a prognostic factor, more powerful than LVEF.30 Moreover, creatine supplementation increases the rate of creatine phosphate resynthesis22 and is associated with enhanced muscular mass, strength, and power.8, 22
Once more, studies on oral supplementation of creatine in CHF have produced conflicting results. In a double‐blind, placebo‐controlled study, a high‐dose (20 g daily) of creatine supplementation to a limited number of CHF patients did not change LVEF but increased energy‐rich phosphagens (total creatine and creatine phosphate) in skeletal muscles as well as muscle strength and endurance.31 However, several reviews8, 22, 32 have concluded that although creatine may be beneficial in CHF patients, its mechanism of action and clinical impact still need to be clarified.
Eufortyn combines creatine and a new water‐soluble CoQ10 formulation, which is known to possess greater bioavailability than the native, lipophilic CoQ10 14 (G. Manini, Scharper Therapeutics; personal communication). We observed a significant increase in peak VO2 and a trend toward an improvement in TWC, combined with a positive effect on SIP physical subscore, which seem to suggest that the combination of the 2 nutraceuticals may be beneficial. Indeed, these results are likely due to the combined pharmacological effects of the 2 molecules on the unhealthy skeletal muscles of CHF patients.
One major limitation of the present study is the fact that we did not measure the plasma concentration of CoQ10 and the muscular concentration of phosphagens. However, as suggested by dose count, our CHF patients had an optimal compliance to trial treatment. Furthermore, a recently published review has affirmed that measurement of plasma levels of micronutrients is of limited importance, as it may not adequately reflect cellular concentrations due to elevated transmembrane gradients.15 Therefore, assessment of biological effects on target cells and tissues would be the only method to determine precisely the efficacy of such substances.15, 33 Further limitations to our study are represented by the relatively small sample size and by assessment of treatment effects short term, and on surrogate outcomes.
Despite these limitations, we believe that our study demonstrated that this commercially available combination of Q‐ter and creatine, which proved to be safe and well tolerated, demonstrated an appreciable improvement in exercise tolerance and HRQL in older CHF patients in NYHA classes II‐III with severe LV systolic dysfunction. Further studies are needed to evaluate long‐term effects of this treatment on functional status, major cardiac events, and prognosis.
Acknowledgements
The authors thank Prof. Alessando Mugelli for his contribution in reviewing the manuscript.
References
- 1. Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112: 1825–1852. [DOI] [PubMed] [Google Scholar]
- 2. Lloyd‐Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. [DOI] [PubMed] [Google Scholar]
- 3. Butler J, Kalogeropoulos A, Georgiopoulou V, et al. Incident heart failure prediction in the elderly: the health ABC heart failure score. Circ Heart Fail. 2008;1:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marchionni N, Di Bari M, Fumagalli S, et al. Variable effect of comorbidity on the association of chronic cardiac failure with disability in community‐dwelling older persons. Arch Gerontol Geriatr. 1996;23:283–292. [DOI] [PubMed] [Google Scholar]
- 5. Masoudi FA, Rumsfeld JS, Havranek EP, et al. Age, functional capacity, and health‐related quality of life in patients with heart failure. J Card Fail. 2004;10:368–373. [DOI] [PubMed] [Google Scholar]
- 6. Rodríguez‐Artalejo F, Guallar‐Castillón P, Pascual CR, et al. Health‐related quality of life as a predictor of hospital readmission and death among patients with heart failure. Arch Intern Med. 2005;165:1274–1279. [DOI] [PubMed] [Google Scholar]
- 7. Heo S, Moser DK, Lennie TA, et al. A comparison of health‐related quality of life between older adults with heart failure and healthy older adults. Heart Lung. 2007;36:16–24. [DOI] [PubMed] [Google Scholar]
- 8. Witte KK, Clark AL, Cleland JG. Chronic heart failure and micronutrients. J Am Coll Cardiol. 2001;37:1765–1774. [DOI] [PubMed] [Google Scholar]
- 9. Vescovo G, Ravara B, Gobbo V, et al. L‐Carnitine: a potential treatment for blocking apoptosis and preventing skeletal muscle myopathy in heart failure. Am J Physiol Cell Physiol. 2002;283: C802–C810. [DOI] [PubMed] [Google Scholar]
- 10. Singh U, Devaraj S, Jialal I. Coenzyme Q10 supplementation and heart failure. Nutr Rev. 2007;65(6 part 1):286–293. [DOI] [PubMed] [Google Scholar]
- 11. Langsjoen PH, Langsjoen AM. Overview of the use of CoQ10 in cardiovascular disease. Biofactors. 1999;9:273–284. [DOI] [PubMed] [Google Scholar]
- 12. Pepe S, Marasco SF, Haas SJ, et al. Coenzyme Q10 in cardiovascular disease. Mitochondrion. 2007;7(suppl):S154–S167. [DOI] [PubMed] [Google Scholar]
- 13. Persky AM, Brazeau GA. Clinical pharmacology of the dietary supplement creatine monohydrate. Pharmacol Rev. 2001;53: 161–176. [PubMed] [Google Scholar]
- 14. Fetoni AR, Piacentini R, Fiorita A, et al. Water‐soluble Coenzyme Q(10) formulation (Q‐ter) promotes outer hair cell survival in a guinea pig model of noise induced hearing loss (NIHL). Brain Res. 2009;1257:108–116. [DOI] [PubMed] [Google Scholar]
- 15. Soukoulis V, Dihu JB, Sole M, et al. Micronutrient deficiencies: an unmet need in heart failure. J Am Coll Cardiol. 2009;54: 1660–1673. [DOI] [PubMed] [Google Scholar]
- 16. Carli F, Corvi Mora P, Canal T. Co‐grinding process for the preparation of a ternary composition. European Patent Office. http://v3.espacenet.com/publicationDetails/biblio?CC=WO&NR=03097012A1&KC=A1&FT=D&date=20031127&DB=EPODOC&locale=en_EP#. 2003.
- 17. Corvi Mora P, Canal T, Ruzzier F, et al. Composition containing micronutrients with improved anti‐oxidant activity and the use thereof. http://www.faqs.org/patents/app/20080219963#ixzz10OdTG2CD. 2008.
- 18. Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation. 2002;106:1883–1892. [DOI] [PubMed] [Google Scholar]
- 19. Marchionni N, Ferrucci L, Baldasseroni S, et al. Item re‐scaling of an Italian version of the sickness impact profile: effect of age and profession of the observers. J Clin Epidemiol. 1997;50:195–201. [DOI] [PubMed] [Google Scholar]
- 20. De Groote P, Isnard R, Assyag P, et al. Is the gap between guidelines and clinical practice in heart failure treatment being filled? Insights from the IMPACT RECO survey. Eur J Heart Fail. 2007;9:1205–1211. [DOI] [PubMed] [Google Scholar]
- 21. Tarnopolsky MA. The mitochondrial cocktail: rationale for combined nutraceutical therapy in mitochondrial cytopathies. Adv Drug Deliv Rev. 2008;60:1561–1567. [DOI] [PubMed] [Google Scholar]
- 22. Witte KK, Clark AL. Micronutrients and their supplementation in chronic cardiac failure: an update beyond theoretical perspectives. Heart Fail Rev. 2006;11:65–74. [DOI] [PubMed] [Google Scholar]
- 23. Molyneux SL, Florkowski CM, George PM, et al. Coenzyme Q10: an independent predictor of mortality in chronic heart failure. J Am Coll Cardiol. 2008;52:1435–1441. [DOI] [PubMed] [Google Scholar]
- 24. Khatta M, Alexander BS, Krichten CM, et al. The effect of coenzyme Q10 in patients with congestive heart failure. Ann Intern Med. 2000;132:636–640. [DOI] [PubMed] [Google Scholar]
- 25. Belardinelli R, Muçaj A, Lacalaprice F, et al. Coenzyme Q10 improves contractility of dysfunctional myocardium in chronic heart failure. Biofactors. 2005;25:137–145. [DOI] [PubMed] [Google Scholar]
- 26. Belardinelli R, Muçaj A, Lacalaprice F et al. Coenzyme Q10 and exercise training in chronic heart failure. Eur Heart J. 2006;27:2675–2681. [DOI] [PubMed] [Google Scholar]
- 27. Soja AM, Mortensen SA. Treatment of congestive heart failure with coenzyme Q10 illuminated by meta‐analyses of clinical trials. Mol Aspects Med. 1997;18(suppl):S159–S168. [DOI] [PubMed] [Google Scholar]
- 28. Sander S, Coleman CI, Patel AA, et al. The impact of coenzyme Q10 on systolic function in patients with chronic heart failure. J Card Fail. 2006;12:464–472. [DOI] [PubMed] [Google Scholar]
- 29. Mortensen SA. Overview on coenzyme Q10 as adjunctive therapy in chronic heart failure. Rationale, design and end‐points of “Q‐symbio”—a multinational trial. Biofactors. 2003;18:79–89. [DOI] [PubMed] [Google Scholar]
- 30. Allard ML, Jeejeebhoy KN, Sole MJ. The management of conditioned nutritional requirements in heart failure. Heart Fail Rev. 2006;11:75–82. [DOI] [PubMed] [Google Scholar]
- 31. Gordon A, Hultman E, Kaijser L, et al. Creatine supplementation in chronic heart failure increases skeletal muscle creatine phosphate and muscle performance. Cardiovasc Res. 1995;30: 413–418. [PubMed] [Google Scholar]
- 32. Witte KK, Nikitin NP, Parker AC, et al. The effect of micronutrient supplementation on quality‐of‐life and left ventricular function in elderly patients with chronic heart failure. Eur Heart J. 2005;26:2238–2244. [DOI] [PubMed] [Google Scholar]
- 33. Xu J, Seo AY, Vorobyeva DA, et al. Beneficial effects of a Q‐ter based nutritional mixture on functional performance, mitochondrial function, and oxidative stress in rats. PLoS One. 2010;5:e10572. [DOI] [PMC free article] [PubMed] [Google Scholar]


