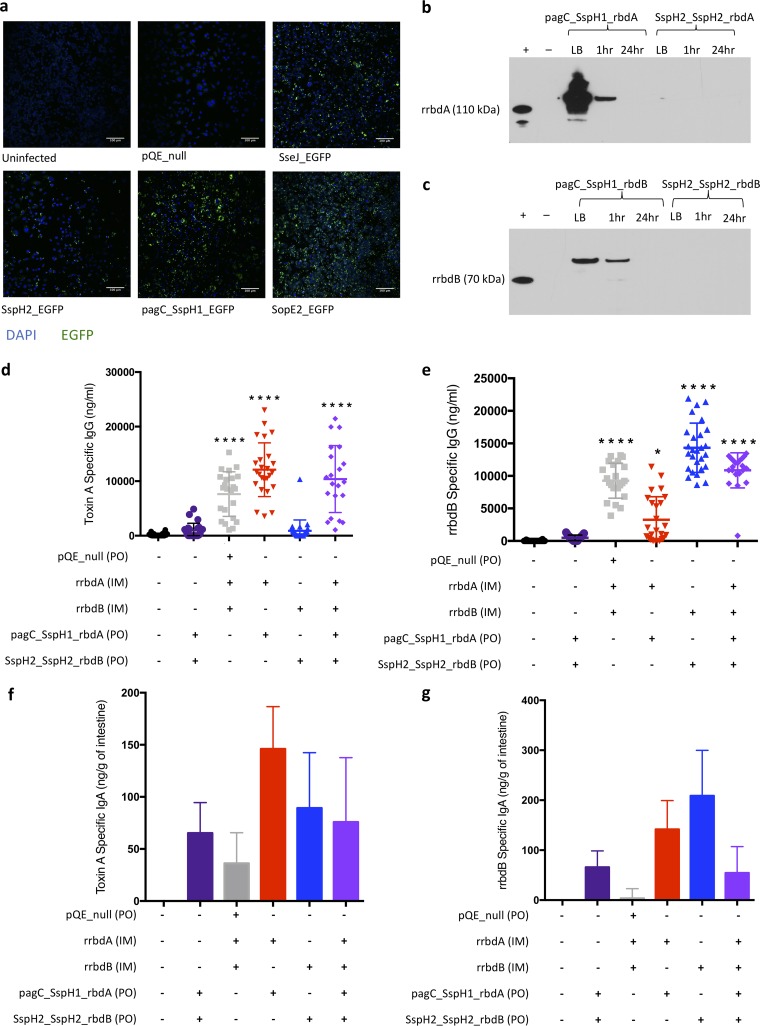
FIG 2.
Transformed YS1646 strains expressed heterologous antigen. (a) EGFP-expressing strains of YS1646 were added to RAW 264.7 macrophages in vitro and visualized 24 h later using a fluorescence microscope. Images are representative of two repeats. (b and c) Antigen expression was examined by Western blotting from YS1646 strains transformed with rbdA (b) and rbdB (c) plasmids. Samples were collected after 16 h of growth in LB and 1 h and 24 h after infection of RAW 264.7 macrophages. Gels were run with a positive control (recombinant RBD antigen, without secretion signals, produced in E. coli), and the film was exposed for 2 min. The increased sizes of the RBDs produced in YS1646 are consistent with the secretion signals that were not cleaved. Mice were immunized with a dose of 10 μg recombinant antigen (rrbdA and/or rrbdB) intramuscularly and three doses of 1 × 109 CFU of antigen expressing YS1646 (pagC_SspH1_rbdA and/or SspH2_SspH2_rbdB) orally every other day. (d and e) Serum was collected at 3 to 4 weeks after vaccination, and toxin A-specific IgG (d) and rrbdB-specific IgG (e) were detected by ELISA (n = 21 to 28, 4 repeats). Data are presented as the mean and standard deviation (SD). (f and g) Intestines were collected 5 weeks after vaccination, and toxin A-specific IgA (f) and rrbdB-specific IgA (g) were detected by ELISA (n = 4 to 5, one repeat). Data are presented as the mean and standard error of the mean (SEM) value from which the mean of the PBS control was subtracted. The Kruskal-Wallis test and Dunn’s multiple-comparison test were used to compare all groups. All P values are by comparison to the PBS control group. *, P < 0.05; ****, P < 0.0001.