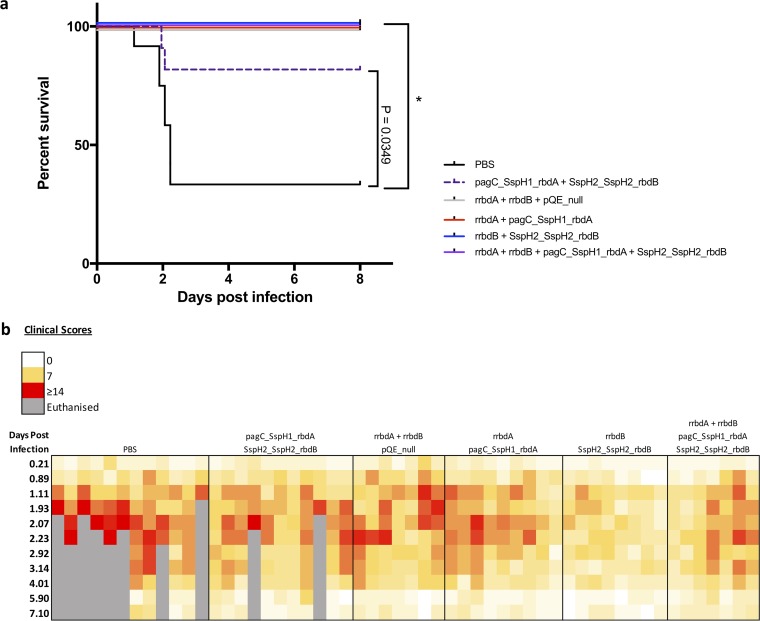
FIG 3.
Vaccination with receptor binding domain (rbd) antigens protected against C. difficile challenge. Mice were immunized with a dose of 10 μg of recombinant antigen (rrbdA and/or rrbdB) intramuscularly and three doses of 1 × 109 CFU of antigen expressing YS1646 (pagC_SspH1_rbdA and/or SspH2_SspH2_rbdB) orally every other day. At 5 weeks after vaccination, mice were challenged p.o. with freshly cultured C. difficile (1.97 × 105 CFU and 1.70 × 107 CFU). Mice were clinically scored 1 to 3 times daily by an observer blind to the treatment. A score of ≥14/20 and/or a >20% loss of the starting body weight was considered the humane endpoint. Survival (a) and clinical scores (b) are shown (n = 7 to 12, 2 repeats). The log-rank (Mantel-Cox) test was used to compare all groups to the PBS control group. Correction of the P value for multiple comparisons was done using the Bonferroni method. *, P < 0.01 compared to the PBS control group.