Figure 5.
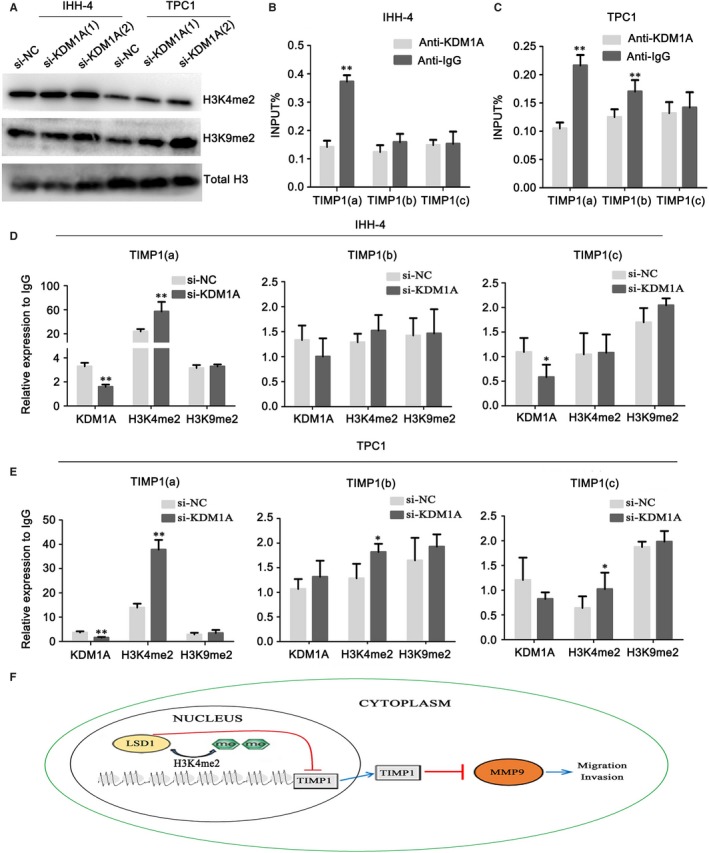
KDM1A removes H3K4me2 at the TIMP1 promoter and represses its promoter activity. (A) Western blot detection of H3K4me2 and H3K9me2 protein levels in IHH‐4 and TPC1 cells after si‐KDM1A or si‐NC transfection. Total histone H3 served as a loading control. (B and C) Analysis of levels of KDM1A occupation at TIMP1 in IHH‐4 (B) and TPC1 cells (C). Schematic drawing of the TIMP1 promoter region and ChIP‐qPCR primer set locations (a, +1051, b, +1626, c, +1868) relative to the TSS (transcriptional start site). TSS was assigned the “+1” position. Chromatin levels of KDM1A were measured by quantitative ChIP. (D and E) Analysis of the levels of occupation of KDM1A, H3K4me2, and H3K9me2 at the TIMP1 gene promoter region after si‐KDM1A or si‐NC transfection in IHH‐4 (D) and TPC1 cells (E). (F) A hypothetical representation of the regulatory pathway underlying KDM1A‐promoted cell migration and invasion. KDM1A represses the TIMP1 gene by removing the epigenetic activation marker H3K4me2. KDM1A‐mediated repression of TIMP1 results in increased MMP9 expression and activation, which are critical for cell migration and invasion. Consequently, MMP9 activation by KDM1A‐mediated repression of TIMP1 contributes to the metastasis of papillary thyroid cancer cells. *P < 0.05; **P < 0.01
