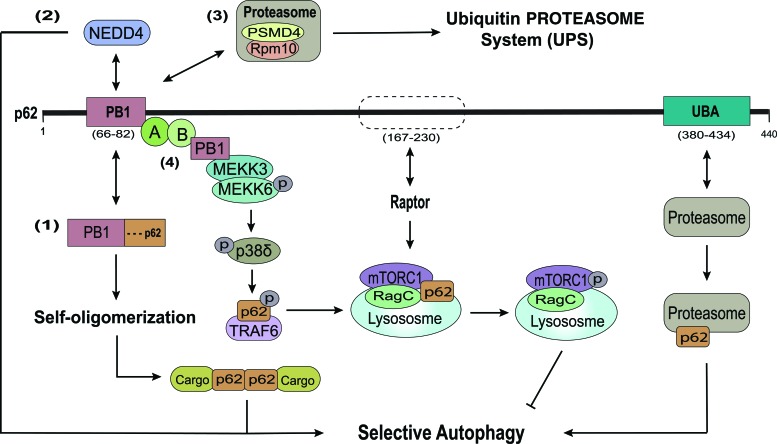
FIG. 3.
Noncanonical domains of p62 involved in selective autophagy. Beside classical LIR and UBA domains mediating selective autophagy, other domains of p62 also play important roles in autophagy as receptors or regulators. (1) The N-terminal PB1 domain is a key component by functioning as an adaptor for the oligomerization of p62 and mediating autophagy initiation in an LIR/UBA-independent manner. (2) The PB1 domain is also recognized and ubiquitinated by the HECT E3 ubiquitin ligase NEDD4, thus enhancing selective autophagic degradation of inclusion bodies. Additional interaction with the 19S proteasomal subunit PSMD4/Rpn10 accelerates delivery and clearance of ubiquitinated cargo to the UPS. (3) The p62 UBA domain also recognizes the proteasome for autophagic degradation. (4) Interaction of PB1 and MEKK3 activates a phosphorylation cascade leading to recruitment of TRAF-6 to p62 TB domain. TRAF-6 promotes translocation of mTOR to lysosomes, its K63 ubiquitination, activation of mTOR signaling, and inhibition of autophagy. A region between the p62 ZZ and TB domains (amino acids 167–230) also participates in lysosomal translocation and activation of mTOR by binding with Raptor and RagC. mTOR, mammalian target of rapamycin; NEDD, neural precursor cell expressed developmentally downregulated protein; UPS, ubiqitin proteasome system. Color images are available online.