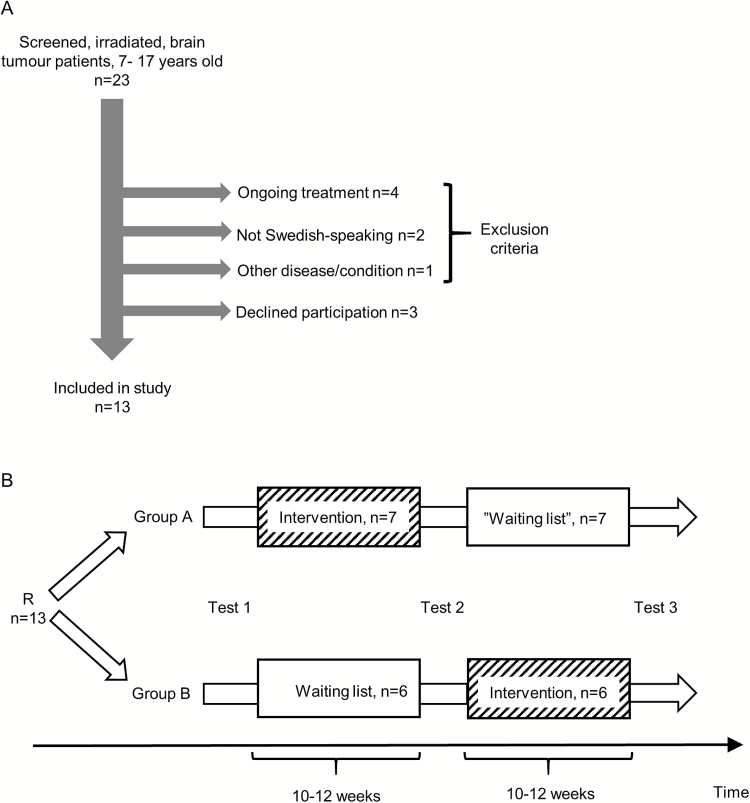
Fig. 1.
A) Inclusion and exclusion. Patients could be included if they had completed brain tumor treatment (including radiotherapy) 1 to 5 years before and were between 7 to 17 years old. Patients were excluded if 1) not in clinical remission or stable disease (ongoing treatment), 2) in a medically unstable situation or suffering from another medical condition making them unable to follow the study protocol (eg, severe mental retardation, severe autism, photosensitive seizures), 3) not Swedish-speaking. No patient had to be excluded due to physical limitations. The patient excluded due to “other disease/condition” had severe autism. B) Study design. After inclusion, participants were randomized (R) to either start with the intervention or waiting list. Tests were performed at 3 time points as indicated in figure. All enrolled subjects completed the study.