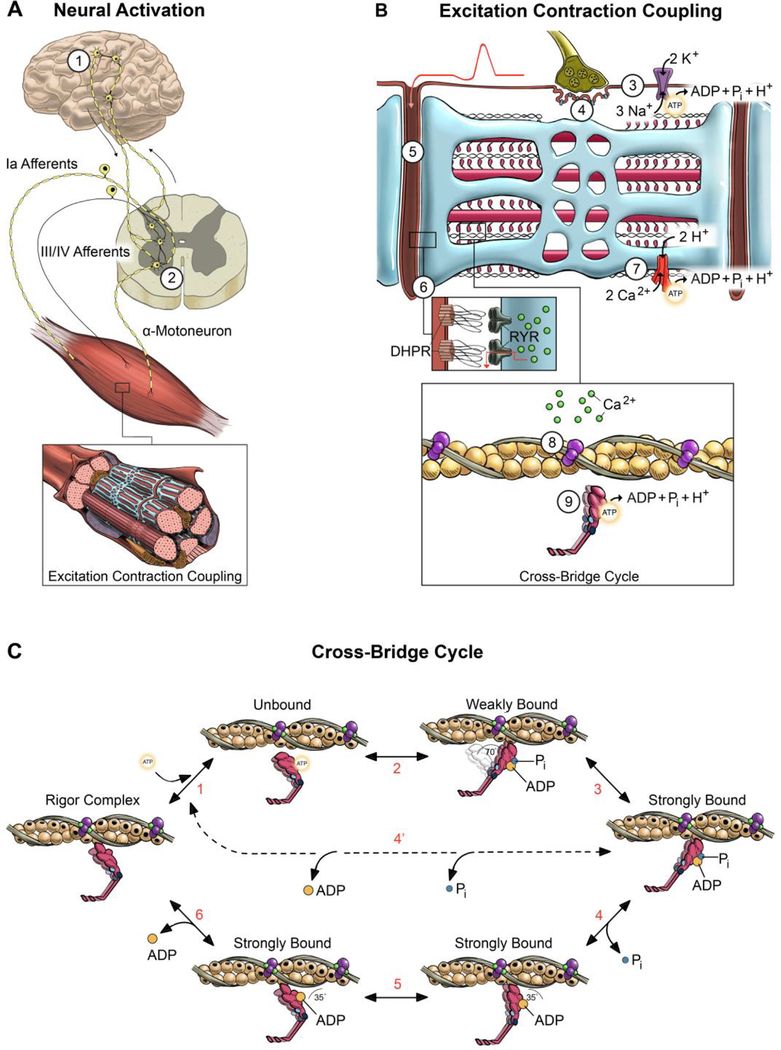
Figure 1. Schematic of potential sites of fatigue along the motor pathway.
Potential sites of fatigue are labeled 1–9 starting with the ability of the nervous system to activate the muscle (A) and progressing to excitation contraction coupling (B) and the cross-bridge cycle (C). During volitional contractions, skeletal muscle is activated via signals originating in the motor cortex (site 1) that are transmitted to the α-motoneurons in the spinal cord (site 2). The output of the polydendritic α-motoneuron is determined by the ensemble synaptic input from thousands of sensory and descending neural pathways, with the group III/IV afferents transmitting signals specific to the metabolic state of the muscle. The propagation of the action potential across the neuromuscular junction (site 4) and into the t-tubule (site 5) is detected by the dihydropyrodine receptor (DHPR) that initiates the release of Ca2+ (Site 6) through the ryanodine receptors (RYR) on the SR membrane. This process requires proper ion gradients and maintained excitability determined, at least in part, by the Na+/K+ pump (site 3) and SR-Ca2+ pump activity (site 7). The binding of Ca2+ to the troponin-tropomyosin complex induces a series of conformational changes in the regulatory proteins (site 8) that allows the interaction of myosin and actin to initiate the power stroke (site 9). Contemporary cross-bridge theory depicted in (C) suggests that the chemo-mechanical transduction of the myosin-actin interaction is partitioned into ~6-strain dependent structural transitions (steps 1–6) that make up the power stroke [32, 53, 72, 73]. Briefly, starting in the rigor complex, ATP binds to the catalytic site on myosin and dissociates myosin from actin (step 1). The hydrolysis of ATP reprimes and cocks the myosin head which attaches to actin in a weakly bound state with the hydrolysis metabolites still bound to the catalytic site (step 2). In the conventional power stroke, the weakly bound cross-bridge transitions to the strongly bound state through unknown mechanisms (step 3). Pi is released from the catalytic site initiating the power stroke where myosin pivots at the light chain domain (step 4). The power stroke continues with an isomerization step where ADP is still bound to the catalytic site (step 5), followed by the release of ADP (step 6). Sites where elevated H+ and Pi disrupt muscle contraction: A majority of the fatigue during high-intensity volitional exercise can be explained by the multifaceted and synergistic effects of increased levels of H+ and Pi. Elevated H+ disrupts contractile function by 1) reducing the sensitivity of the myofilaments to Ca2+ (site 8), 2) decreasing force of the cross-bridge by inhibiting the low- to high-force transition (step 3), and 3) slowing shortening velocity by inhibiting the ADP isomerization step (step 5) and/or the release of ADP (step 6). Elevated Pi disrupts contractile function by 1) decreasing the free [Ca2+] available for release from the SR (site 6), 2) reducing the sensitivity of the myofilaments to Ca2+ (site 8), and 3) decreasing force by inducing an unconventional power stroke where myosin dissociates from actin early in the high-force state prior to the release of Pi and ADP (step 4’).