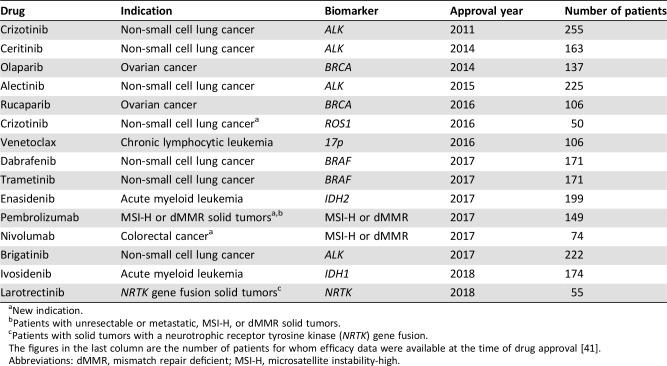
Table 3. Drug‐diagnostic combinations that have obtained FDA approval based on efficacy data from small phase I/II trials.
New indication.
Patients with unresectable or metastatic, MSI‐H, or dMMR solid tumors.
Patients with solid tumors with a neurotrophic receptor tyrosine kinase (NRTK) gene fusion.
The figures in the last column are the number of patients for whom efficacy data were available at the time of drug approval [41].
Abbreviations: dMMR, mismatch repair deficient; MSI‐H, microsatellite instability‐high.