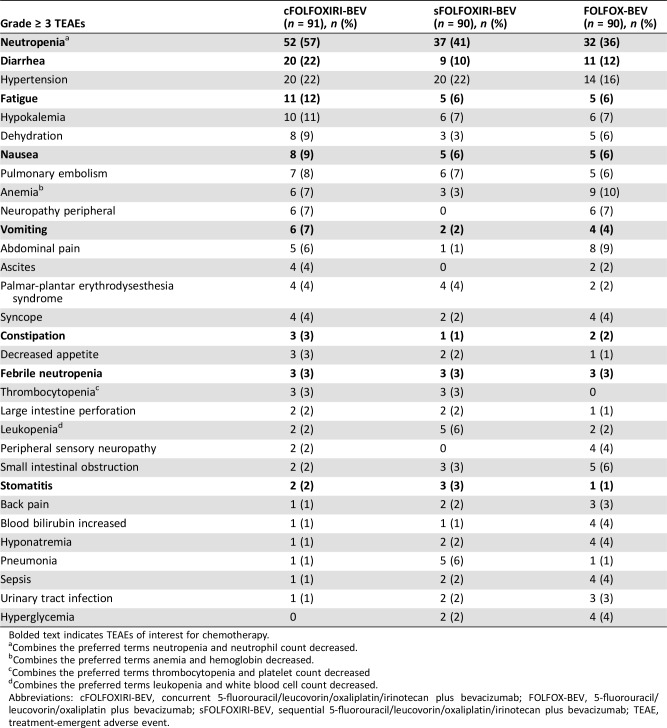
Table 5. Grade ≥ 3 TEAEs occurring in at least five patients (safety population).
Bolded text indicates TEAEs of interest for chemotherapy.
Combines the preferred terms neutropenia and neutrophil count decreased.
Combines the preferred terms anemia and hemoglobin decreased.
Combines the preferred terms thrombocytopenia and platelet count decreased
Combines the preferred terms leukopenia and white blood cell count decreased.
Abbreviations: cFOLFOXIRI‐BEV, concurrent 5‐fluorouracil/leucovorin/oxaliplatin/irinotecan plus bevacizumab; FOLFOX‐BEV, 5‐fluorouracil/leucovorin/oxaliplatin plus bevacizumab; sFOLFOXIRI‐BEV, sequential 5‐fluorouracil/leucovorin/oxaliplatin/irinotecan plus bevacizumab; TEAE, treatment‐emergent adverse event.