The treatment of choice for patients with carcinoid syndrome is based on somatostatin analogs. This brief communication presents the case of a patient with a neuroendocrine tumor who participated in the TELESTAR trial and reports on the effectiveness of telotristat ethyl for improved control of diarrhea associated with carcinoid syndrome refractory to somatostatin analogs. Moreover, the article explains the rationale for potential antitumor effects related to inhibition of serotonin synthesis in neuroendocrine tumors.
Abstract
In this article, we propose, based on a clinical case, the potential antitumor effect related to the inhibition of serotonin in neuroendocrine tumors (NETs). Currently, the only drug that exists for the symptomatic treatment of carcinoid syndrome refractory to somatostatin analogues is telotristat, based on its pivotal study, the TELESTAR trial. Based on the existing preclinical rationale, it seems that the inhibition of serotonin may have an antitumoral role in NETs. Briefly, serotonin may act as an autocrine growth factor of NETs, and it may also play an immunomodulatory role by enhancing macrophage polarization to an immunotolerant M2 phenotype. To our knowledge, this rationale for the possible antitumor effect of serotonin in NETs has not yet been published in the literature.
Introduction
Carcinoid syndrome represents a group of symptoms such as diarrhea or flushing, along with, in the long term, peritoneal and cardiac valvular fibrosis [1]. Its incidence reaches up to 19% in patients with neuroendocrine tumors (NETs). Survival outcomes seem to be worse in patients who develop carcinoid syndrome than in patients who do not [2].
Serotonin plays a central role in the development and maintenance of carcinoid syndrome. Physiologically, it is secreted by enterochromaffin cells in the gastrointestinal tract to regulate motility, secretion, and inflammation functions. Carcinoid tumors are usually derived from enterochromaffin cells, usually of the midgut, and often release large amounts of serotonin [1], [3].
The treatment of choice for patients with carcinoid syndrome is based on somatostatin analogs (SSAs). Initially, SSAs are able to control the symptoms derived from carcinoid syndrome, but approximately 71% of the patients treated become refractory after 36 months of SSA treatment [4].
Telotristat ethyl is an inhibitor of the peripheral serotonin synthesis and acts by inhibiting tryptophan hydroxylase, the rate‐limiting enzyme in the conversion of tryptophan to serotonin. Telotristat ethyl has demonstrated its effectiveness in the phase III TELESTAR trial by improving the control of diarrhea associated with carcinoid syndrome refractory to SSA [5]. This study did not include the analysis of survival or antitumoral outcomes, so we do not have, at the moment, any clinical evidence of the activity of serotonin inhibition beyond the reduction in the number of bowel movements in patients with carcinoid syndrome.
Summary
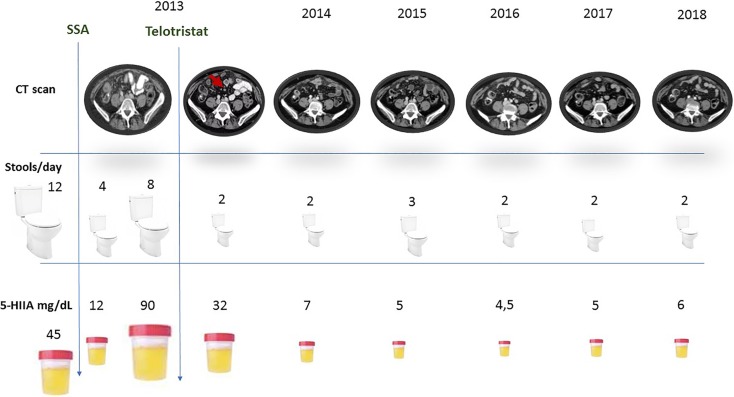
We present the case of a 67‐year‐old woman who came to the emergency department for abdominal pain. Computed tomography was performed, and an ileal thickening along with peritoneal metastases and a single hepatic lesion was identified. Hepatic and peritoneal metastases were biopsied, showing metastatic infiltration of a well‐differentiated NET (Ki67 = 3% in both sites). In addition, the patient presented a carcinoid syndrome with diarrhea consisting of 8–12 stools per day and 3–4 flushing episodes daily. Urinary 5‐hydyroxyindoleacetic acid (5‐HIAA) at diagnosis was 45 mg/24 hours.
In February 2013, the patient began octreotide LAR 30 mg/28 days and obtained a significant clinical benefit with a reduction in the number of stools, up to four daily, and a decrease in the number of flushing episodes. The urinary 5‐HIAA also experienced an important reduction below 12 mg/24 hours. However, in August 2013, the patient suffered from a worsening in the carcinoid syndrome symptoms and an increase in the urinary 5‐HIAA (90 mg/24 hours). This clinical worsening was associated with a radiological progression by the identification of new lesions in the peritoneum. At that time, the patient was offered to participate in the TELESTAR trial. She accepted and initiated treatment with telotristat 250 mg three times per day, in addition to octreotide LAR 30 mg/28 days.
During the first month of treatment, the patient presented a significant clinical improvement, with a decrease in the number of bowel movements (maximum two per day) and almost complete resolution. At the present time, the patient presents symptomatic relief of the carcinoid syndrome symptoms with important improvement in the quality of life. She maintains urinary 5‐HIIA levels below 5 mg/24 hours. The patient has achieved peritoneal and hepatic stabilization, with no appearance of new lesions during the last 5 years. The treatment with telotristat was well tolerated with grade 1 nausea as the only related adverse event (Fig. 1).
Figure 1.
Patient clinical, radiological, and biochemical behavior.
Abbreviations: 5‐HIIA, 5‐hydyroxyindoleacetic acid; CT, computed tomography; SSA, somatostatin analog.
Discussion
In relation to this case, there are still relevant issues to be solved about the role of telotristat in NET management. We are not sure about the long‐term efficacy of telotristat in the prevention and treatment of chronic carcinoid syndrome effects. Because telotristat is a relatively new drug, there is not enough follow‐up to determine its implication in these long‐term complications. Furthermore, the potential role of serotonin as a tumor growth factor in NETs and its capacity to modify the tumor microenvironment remains to be clarified.
Preclinical data have demonstrated that serotonin (5‐HT) may have activity as an autocrine growth factor to stimulate proliferation of lung and gastrointestinal NET cells through alterations in extracellular signal‐regulated kinase and c‐Jun N‐terminal kinase signaling [6]. The authors suggested that tumor cell proliferation was inhibited by ketanserin and ondansetron as well as 7‐hydroxytryptophan, all of them considered competitive or antagonists of serotonin. In addition, research in human BON cell lines, a model that has been used to study pancreatic NET cell biology, showed that 5‐HT stimulates cell proliferation and acts as an autocrine growth factor through specific receptors [7].
Furthermore, serotonin is involved in macrophage polarization mechanisms. There are different macrophages subpopulations that have been defined, among which the M1‐macrophages are involved in CD4 Th1 cytotoxic polarization and tumor control response and the M2‐macrophages are associated with tumor immune tolerance and angiogenesis [8]. De las Casas‐Engel et al. have shown that macrophages have a differential expression of serotonin receptors on their surface depending on the polarization [9]. Thus, when the macrophage has reached an M2‐polarization, investigators found an expression higher than 500‐fold of the mRNA that encodes for the 5HT2B receptor than in the macrophage with M1‐polarization. Also, they demonstrated, by Western blot analysis, greater expression of 5HT2B in cell lysates by M2‐macrophages in comparison with M1‐macrophages. Functional assays have evaluated the expression of genes related to the macrophage polarization to M2‐phenotype. By quantitative reverse transcription polymerase chain reaction (qRT‐PCR), the authors demonstrated that the addition of serotonin increased the expression of genes, such as SERPINB2/COL23A1/THBS1/STAB1, while reducing those related to M1‐phenotype polarization, such as INHBA/CCR2/SERPINE1/MMP12 [9].
When culturing monocytes and adding serotonin together with different serotonergic antagonists of both, the 5HT2B/5HT7 receptors, a decrease in the genes related to M2‐macrophage polarization is observed with an increase of those related to the M1‐polarization, by the qRT‐PCR technique [9].
The case presented here is an example of a long survivor with adequate clinical and radiological control of the disease under treatment with telotristat.
Conclusion
There are some data that could suggest the potential antitumor activity of serotonin inhibition: On the one hand, the role in macrophage phenotype polarization toward an M1‐phenotype involved in the tumor necrosis factor alpha/interleukin 12 secretion for a cytotoxic activity, and on the other, the role in NET cells proliferation by inhibiting the autocrine effect of serotonin in cell growth, as has been already demonstrated in preclinical models. The hypothetical antitumor activity of telotristat as a peripheral inhibitor of serotonin synthesis needs to be tested in preclinical models and, if confirmed, in prospective clinical studies.
Disclosures
Enrique Grande: Pfizer, Bristol‐Myers Squibb, Ipsen, Roche, Eisai, Eusa Pharma, Merck Sharp & Dohme, Sanofi‐Genzyme, Adacap, Novartis, Pierre Fabre, Lexicon, Celgene (C/A, SAB), Pfizer, AstraZeneca, MTEM/Threshold, Roche, Ipsen, Lexicon (RF). Teresa Alonso‐Gordoa: Pfizer, Ipsen, Bristol‐Myers Squibb, Roche, Eisai, Merck Sharp & Dohme, Sanofi‐Genzyme, Novartis, AstraZeneca (C/A), Roche (RF). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Molina‐Cerrillo J, Alonso‐Gordoa T, Martínez‐Sáez O et al. Inhibition of peripheral synthesis of serotonin as a new target in neuroendocrine tumors. The Oncologist 2016;21:701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halperin DM, Shen C, Dasari A et al. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: A population‐based study. Lancet Oncol 2017;18:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modlin IM, Kidd M, Latich I et al. Current status of gastrointestinal carcinoids. Gastroenterology 2005;128:1717–1751. [DOI] [PubMed] [Google Scholar]
- 4.Beaumont JL, Cella D, Phan AT et al. Comparison of health‐related quality of life in patients with neuroendocrine tumors with quality of life in the general US population. Pancreas 2012;41:461–466. [DOI] [PubMed] [Google Scholar]
- 5.Kulke MH, Hörsch D, Caplin ME et al. Telotristat ethyl, a tryptophan hydroxylase inhibitor for the treatment of carcinoid syndrome. J Clin Oncol 2017;35:14–23. [DOI] [PubMed] [Google Scholar]
- 6.Drozdov I, Kidd M, Gustafsson BI et al. Autoregulatory effects of serotonin on proliferation and signaling pathways in lung and small intestine neuroendocrine tumor cell lines. Cancer 2009;115:4934–4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grozinsky‐Glasberg S, Shimon I, Rubinfeld H. The role of cell lines in the study of neuroendocrine tumors. Neuroendocrinology 2012;96:173–187. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A, Marchesi F, Malesci A et al. Tumour‐associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 2017;14:399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de las Casas‐Engel M, Domínguez‐Soto A, Sierra‐Filardi E et al. Serotonin skews human macrophage polarization through HTR2B and HTR7. J Immunol 2013;190:2301–2310. [DOI] [PubMed] [Google Scholar]