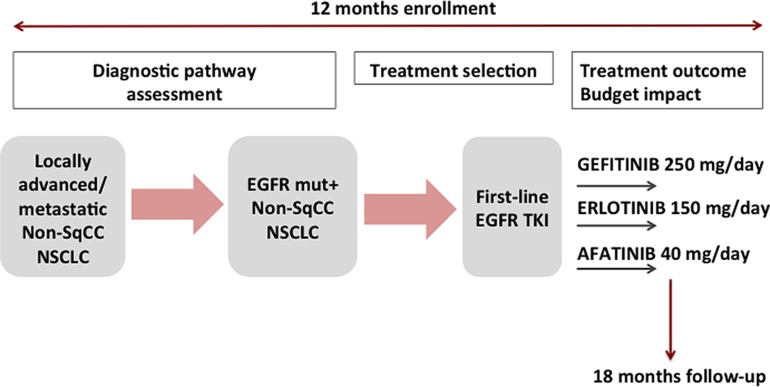
Figure 1.
Study design. Patients with nonsquamous NSCLC were included in the study. The recruitment lasted 12 months. The follow‐up time lasted 18 months from the inclusion of the last patient. Diagnostic pathway was monitored for all patients with nonsquamous NSCLC, whereas treatment outcome and budget impact analysis were assessed in patients with EGFR‐mutant NSCLC.
Abbreviations: EGFR, epidermal growth factor receptor; mut+, mutation positive; non‐sqCC, nonsquamous; NSCLC, non‐small cell lung cancer; TKI, tyrosine kinase inhibitor.