Figure 2.
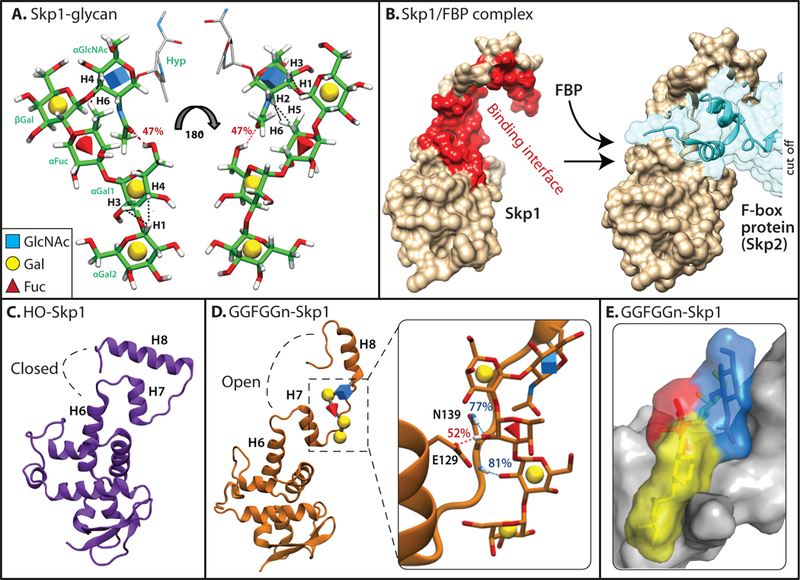
The Dictyostelium pentasaccharide and its effect on Skp1 conformation. (a) The sequence and linkages of the glycan were determined by analysis of the 13C-sugar labeled glycopeptide and confirmed on 13C-sugar labeled versions of Gal-Gal-Fuc-Gal-GlcNAc-Skp1 (GGFGGn-Skp1). Its conformation was inferred from calculation of its lowest energy state (Glycam), molecular dynamics simulations, and NMR studies of 13C-GGFGGn-Skp1. Black dashed lines represent NOEs observed from NMR analysis of GGFGGn-Skp1; red dashed line represents a hydrogen bond observed during MD simulations. (b) The conformation of unmodified human Skp1 in a crystallographic complex (PDB 2ASS) with the FBP Skp2 (at right). The largely hydrophobic binding interface is highlighted in red (left) using PISA analysis in the excerpted surface rendition at the left. The highly conserved C-terminal region (upper half) can adopt at least two different conformations with other FBPs [38–40]. Images were generated using Chimera. (c) Back-side view (relative to panel c) of the average conformation of Dictyostelium HO-Skp1 during MD simulations initiated from the structure in panel c. (d) Average conformation of GGFGGn-Skp1, with the inset showing hydrogen bonds which are present during over 50% of the simulation time. (e) Space-filling model of glycosylated region of Skp1, depicting van der Waals contacts of the glycan with the polypeptide. Panels a, c, d, and e are modified from ref. 41⦁⦁.
